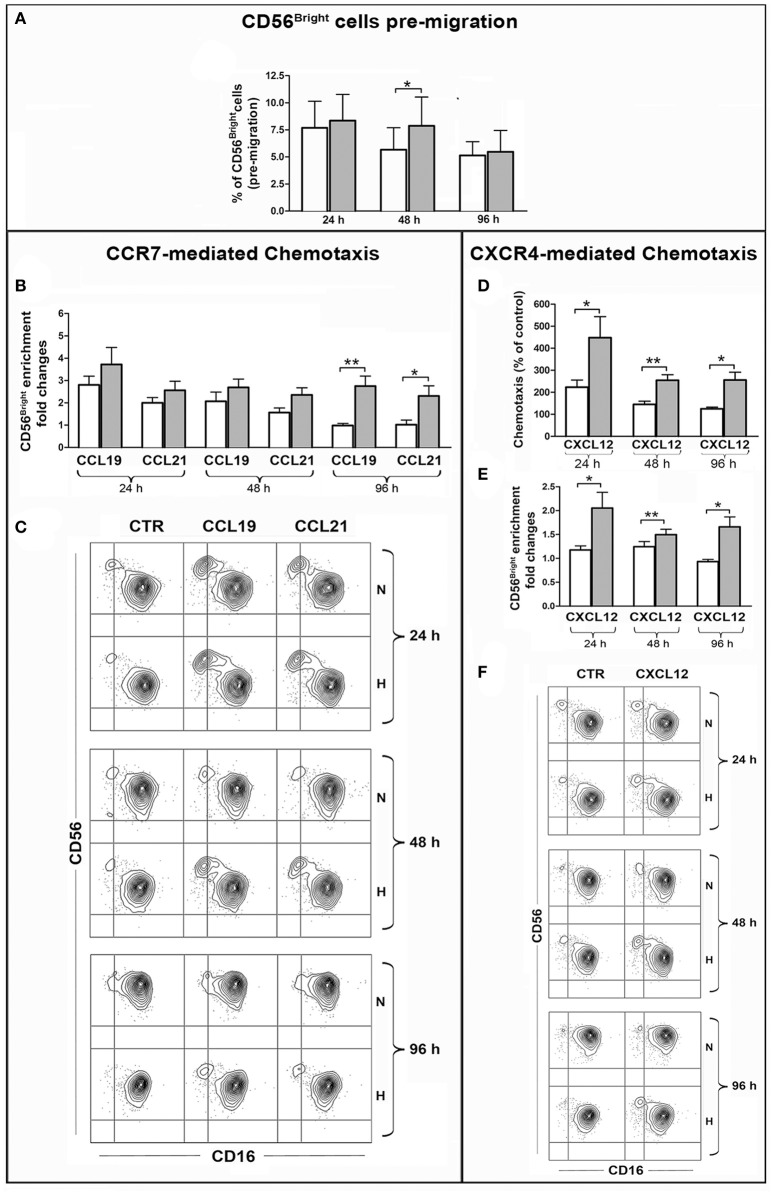
Figure 6.
Effect of hypoxia on chemotaxis of PB-NK cells and their CD56bright/CD56dim subset to CXCL12, CCL19, CCL21. PB-NK cells were cultured in the presence of IL-2 for 24, 48, or 96 h under normoxic or hypoxic conditions and analyzed by FACS for the combined expression of CD56 and CD16 markers in order to assess the percentage of the CD56brightCD16dim/neg cells before migration (A). Cells were then assessed for chemotaxis to the indicated chemokines under normoxic conditions for 2 h. Migrated cells were collected from the lower migration chamber compartments and counted or analyzed by FACS for the combined expression of CD56 and CD16 markers. The specific chemotactic response of CD56brightCD16dim/neg NK cells to CCL19, CCL21 (B), and CXCL12 (E) was assessed as enrichment of this cell subset within migrated cells. The enrichment was calculated as fold increase of the CD56brightCD16dim/neg cell percentage within cells migrated to specific chemokines as compared to the CD56brightCD16dim/neg cell percentage within spontaneously migrated cells (see section Materials and Methods for details). (C,F) Representative experiments showing the enrichment of CD56brightCD16dim/neg NK cells within cells migrated in response to CCL19, CCL21 (C) or CXCL12 (F) as compared to cells that spontaneously migrated in the lower compartment in the absence of stimuli (CTR). (D) Specific chemotactic response to CXCL12 of the whole PB-NK cell population cultured under normoxic or hypoxic conditions. In (B,D,E) white and gray bars indicate data from NK cells cultured under normoxic or hypoxic conditions, respectively and represent the mean ± SEM of 6 independent experiments. * p < 0.05, ** p < 0.01.