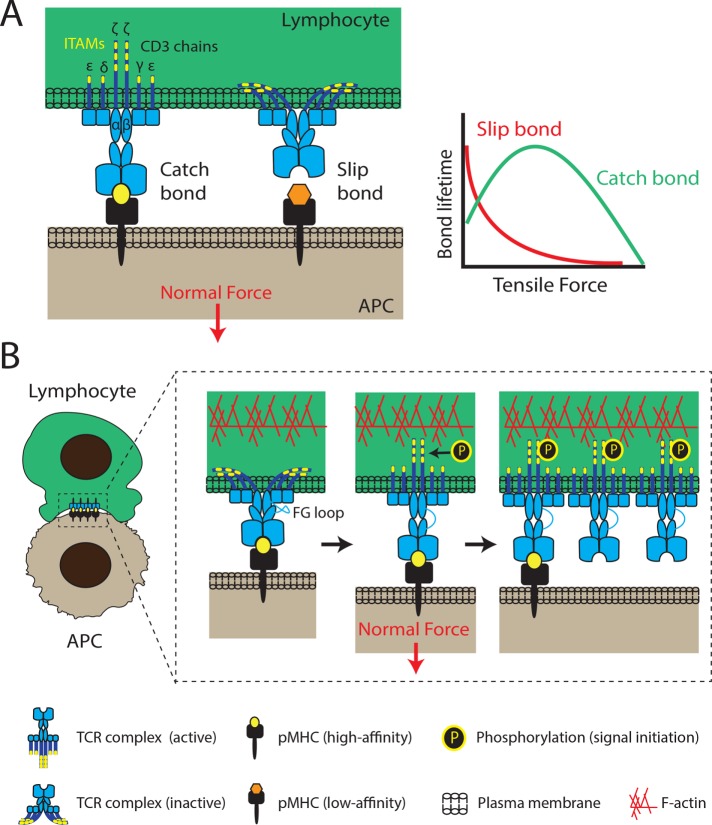
FIGURE 1:
Mechanotransduction through the T-cell receptor. (A) The T-cell receptor complex consists of an αβ heterodimer that is noncovalently associated with ITAM-containing CD3 chains (εγ, εδ, and ζζ dimers). TCRs interact tightly with high-affinity pMHCs, forming catch bonds that are characterized by lifetimes that increase under load. Above a specific force threshold, lifetimes decrease. Longer TCR–pMHC interactions are more likely to lead to successful signal initiation and T-cell activation. Conversely, TCR interactions with low-affinity peptides exhibit slip-bond behavior, rupturing easily under low tensile forces. (B) Receptor deformation model of TCR activation. Force applied to the TCR upon pMHC binding triggers the unfolding of the FG loop region of the TCR and the exposure of its ITAMs (yellow bands) for phosphorylation and initiation of downstream signaling. FG loop unfolding facilitates extension of the TCR and its catch-bond behavior. Additionally, conformational changes of one TCR complex can propagate to its neighbors, producing clusters of active TCR complexes to amplify signaling. Both normal and tangential forces have been shown to initiate TCR signaling (normal forces shown here), with the contribution of each force component still under investigation. The F-actin cytoskeleton is thought to play a major role in both force generation and TCR clustering.