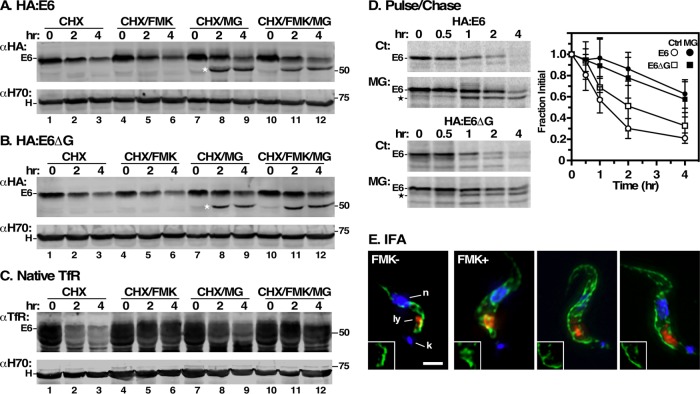
FIGURE 6:
Turnover of E6 reporters. (A, B) HA:E6- and HA:E6ΔG-expressing cell lines were harvested after TfR RNAi induction (24 h) and cultured with cycloheximide (CHX, 100 μg/ml) to stop protein synthesis. The cells were also treated with FMK024 (FMK, 20 μM) or MG132 (MG, 25 μM) as indicated. Samples (107 cell equivalents) were collected at 0, 2, or 4 h and immunoblotted with anti-HA (αHA) or anti-Hsp70 (αH70). Assay measures loss of steady state reporter as a function of time. The mobility of the MG132-protected species is indicated by a star. (C) Turnover of native E6 was assessed in the HA:E6 cell line without silencing exactly as in panels A and B. (D) Turnover of HA:E6 and HA:E6ΔG reporters was assayed by pulse/chase (15 min/4 h) radiolabeling in cells post–TfR RNAi (24 h). As indicated, cells were either untreated (Ct) or treated with MG132 (MG, 25 μM). Typical phosphor images of anti-HA immunoprecipitates at the indicated chase times are presented (107 cell equivalents/lane). The mobilities of the MG132-protected E6 species are indicated by a star. Loss of the HA:E6 or HA:E6ΔG reporter during the chase period was quantified (mean ± SD, n = 5). (E) IFA was performed post–TfR RNAi induction with the HA:E6-expressing cell line, with and without FMK024 treatment (20 μM, 4 h). Staining of fixed permeabilized cells was performed with mAb anti-HA (green) and with rabbit anti-TbCatL (red). Deconvolved three-channel summed stack images with DAPI staining (blue) are presented. Insets are the single channel anti-HA images in the region of the lysosome. Bar, 2 μm.