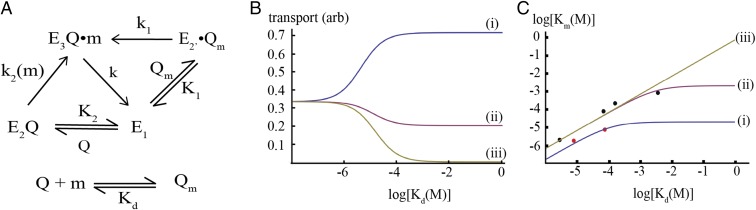
Fig. 9.
Kinetic analysis of canonical and noncanonical substrate uptake by ABC transporters. (A) Kinetic scheme modeling canonical and noncanonical pathways in substrate import by the transporter, E, where E1, E2, E2′, and E3 represent different states of the transporter as shown in Fig. 8. The methionine ligand (m) binds to Q to form the liganded species Qm with a dissociation constant Kd. Qm and Q can bind to E1 to form E2′Qm and E2Q, with effective dissociation constants K1 and K2, respectively. Transport occurs from the state E3Q•m that is generated either by isomerization of E2′Qm with the first-order rate constant k1 or by binding of m to E2Q with a second-order rate constant k2. The steady-state solution to this scheme for the case where E, E2′Qm, E2Q, Q, and Qm are at equilibrium is described in Materials and Methods. (B) Dependence of the transport rate for a substrate at 10 µM concentration on various parameters of the kinetic scheme in A, as a function of the Kd for binding of substrate to the SBP. Curves i, ii, and iii correspond to k2 = 5,000, 500, and 0 M−1⋅s−1, with Qtot = 10−4 M, K1 = K2 = 10−4 M, and k = k1 = 0.01 s−1. For substrates with high affinity to the SBP (Kd < ∼10−6 M) the models are equivalent, whereas for more weakly bound substrates (Kd > ∼10−4 M) the transport rate depends critically on the value of k2. Depending on the value of k2, the rate of transport of a weakly bound substrate can exceed that of a substrate that binds tightly to the SBP (compare curves i and ii). When k2 = 0 (curve iii), transport does not occur under these conditions, which corresponds to the case where substrate is only delivered to E1 by binding to the SBP. (C) Dependence of the Km for transport on the dissociation constant Kd for binding of substrate to the SBP. The red and black circles represent experimental data points for the methionine (this study) and maltose transporter (30), respectively. The curves are generated with the following parameters: i, k = 0.01 s−1, k1 = 0.1 s−1, and k2 = 1,000 M−1⋅s−1; ii, k = 0.1 s−1, k1 = 0.1 s−1, and k2 = 100 M−1⋅s−1; and iii, k = 0.1 s−1, k1 = 0.1 s−1, and k2 = 0 M−1⋅s−1, with Qtot = 10−4 M and K1 = K2 = 10−4 M for all curves. Curves were not explicitly fit to the experimental data but reflect parameter values that approximate the data. For this kinetic scheme, Km reaches a plateau as Kd increases, and depending on the parameter values, this plateau can be tuned to the physiological concentration range. For curve iii, where substrate does not bind to E2Q, a plateau region is not reached, so substrates with poor affinities for Q (high Kd) would have correspondingly high Km values.