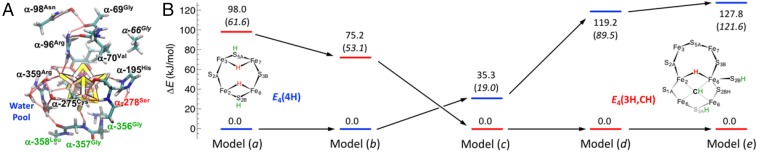
Fig. 4.
Effect of the enzymatic environment around FeMo-co on the relative stability of the bridging hydride state E4(4H) and C-protonated state E4(3H,CH). (A) Models used in the present study, with the labels of the residues sequentially removed from the full model (a): highlighted in blue [model (b)], green [model (c)], red [model (d)], and black [model (e)], respectively (SI Appendix, Fig. S1). (B) Corresponding relative electronic energy (no polarizable continuum corrections applied) as obtained from DFT/BP86 and DFT/B3LYP level of theory (values in parentheses).