Scheme 3.
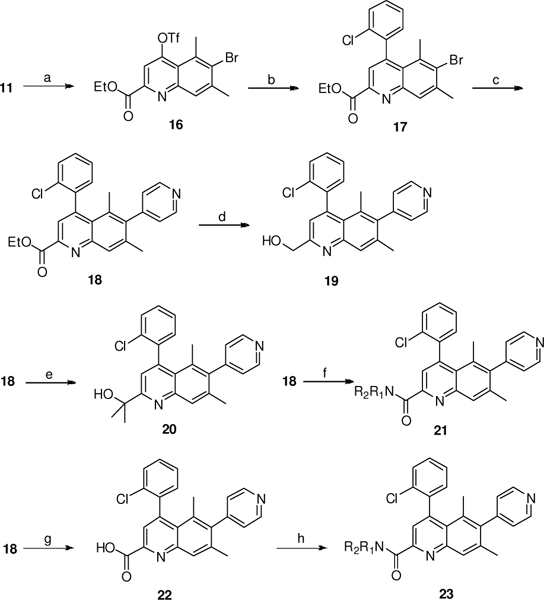
Synthesis of C2 substituted quinoline analogs 18–23. Reagents and conditions: a) Tf2O, Et3N, CH2Q2; b) o-ClC6H4B(OH)2, Pd(PPh3)4, Et3N, dioxane, 100 oC, 60%, 2 steps; c) XPhos-Pd-G2, 4-pyridyl boronic acid, Na2CO3, dioxane/H2O, 60 oC, 66%; d) LiAlH4, THF, 52%; e) MeMgBr, THF, −20 oC, 35%; f) R1R2NH, MeOH, 10–70%; g) NaOH, THF/H2O, 70%; h) R1R2NH, EDCI, HOBt, iPr2EtN, DMF, 11–45%
