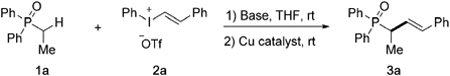
Table 1:
Condition optimization for α-vinylation of 1a.[a]
| Entry | Base | Catalyst | 3aa, Yield [%][b] |
|---|---|---|---|
| 1 | KOH | CuOTf·1/2 PhMe | 0 |
| 2 | KOtBu | CuOTf·1/2 PhMe | 0 |
| 3 | NaOMe | CuOTf·1/2 PhMe | 0 |
| 4 | KHMDS | CuOTf·l/2 PhMe | 0 |
| 5 | n-BuLi | CuOTf·1/2 PhMe | 0 |
| 6 | Mg(tmp)Cl·LiCl | CuOTf·1/2 PhMe | 47 |
| 7 | Zn(tmp)2·(LiCl)2 | CuOTf·1/2 PhMe | 29 |
| 8 | Zn(tmp)Cl·LiCl | CuOTf·1/2 PhMe | 83 |
| 9 | Zn(tmp)Cl·LiCl | CuCl | 80 |
| 10 | Zn(tmp)Cl·LiCl | CuBr | 81 |
| 11 | Zn(tmp)Cl·LiCl | Cu[CH3CN]4PF6 | 68 |
| 12 | Zn(tmp)Cl·LiCl | Cu(OAc)2 | 66 |
| 13 | Zn(tmp)Cl·LiCl | Cu(OTf)2 | 92 (86)[c] |
| 14[d] | Zn(tmp)Cl·LiCl | Cu(OTf)2 | (87)[c] |
| 15 | Zn(tmp)Cl·LiCl | - | trace |
Conditions: 1a (0.2 mmol), base (0.3 mmol), 2a (0.24 mmol), catalyst (10 mol%), THF (2 mL), RT.
Yields determined by 1H-NMR with CH2Br2 as an internal standard.
Yields of isolated products.
Reaction run at 50°C.
