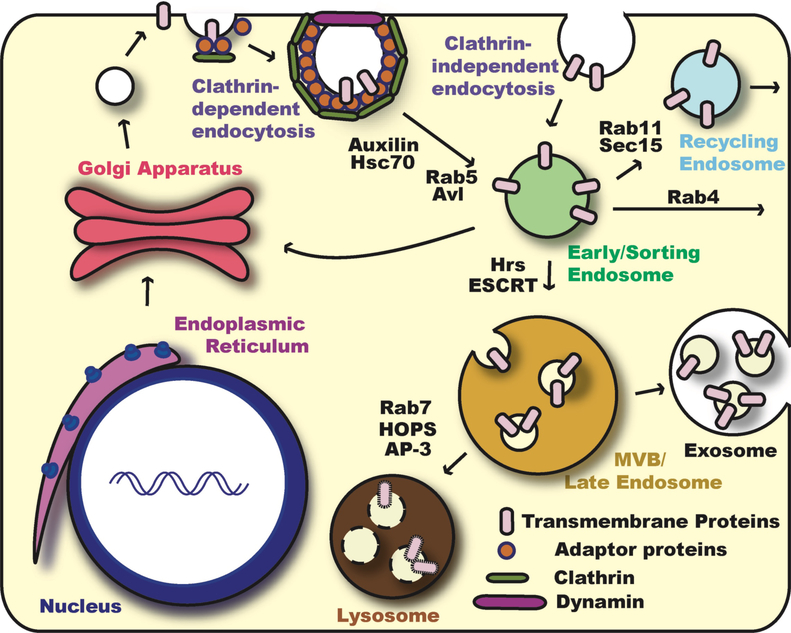
Fig. 1. Overview of Endocytosis and Vesicle Trafficking.
Transmembrane proteins are made in the ER and traffic through the Golgi apparatus to reach the plasma membrane. From the cell surface, these proteins can re-enter the cell via various endocytosis pathways. Clathrin-dependent endocytosis is usually referred to as “canonical endocytosis”. Clathrin adaptor proteins, such as the AP-2 complex, recruit clathrin and cargo transmembrane proteins to the site of endocytosis. The clathrin-coated endocytic vesicle is pinched off by the action of Dynamin GTPase and the clathrin coat is then removed by molecular chaperone Hsc70 via the assistance of Auxilin. On the other hand, endocytosis can also occur without clathrin and is referred to as “non-canonical endocytosis” or “Clathrin-independent endocytosis”. After endocytosis, small GTPase Rab5 and SNARE protein Avalanche (Avl) mediate the fusion of endocytic vesicles with the early/sorting endosome. From the early endosome, endocytosed proteins can recycle back to the plasma membrane directly in a Rab4-dependent manner or indirectly through the recycling endosome in a Rab11-dependent manner. Alternatively, they can return back to the Golgi, or travel to the late endosome and lysosome for degradation. Proteins destined for degradation are sorted into Rab7-positive late endosome or multi vesicular bodies (MVB). Packaging of transmembrane proteins into intraluminal vesicles is mediated by the ESCRT complexes. In certain cell contexts, MVB can secrete their contents to extracellular regions. These secreted MVBs are referred to as exosomes. Finally, through HOPS and AP3 complexes, MVB/late endosomes fuse with the lysosome and transmembrane proteins are degraded by proteases and acid hydrolases.