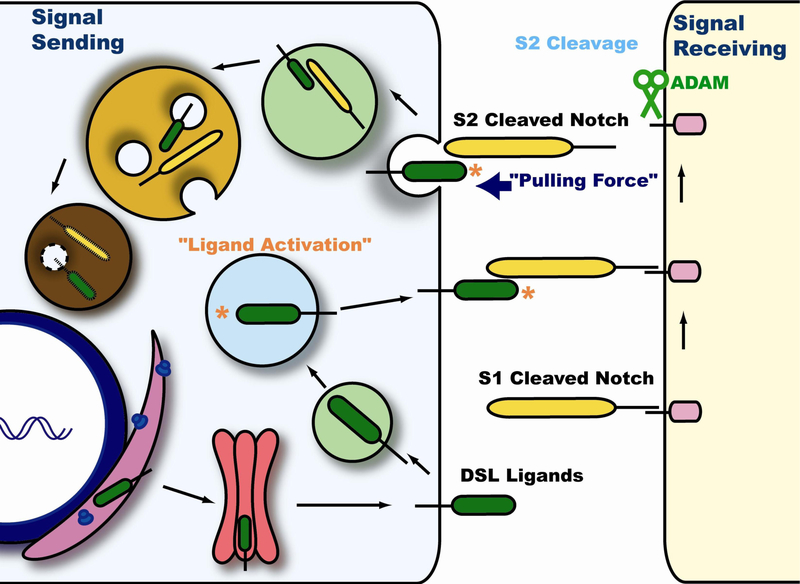
Fig. 2. Endocytosis and Trafficking of DSL Ligands in the Signal Sending Cell.
DLS ligands are synthesized in the ER and traffics to the cell surface through the secretory pathway. Endocytosis is required in the signal sending cell for activation of the canonical Notch signaling pathway, but there are two non-mutually exclusive hypotheses to explain how endocytosis promotes DSL ligand activity. In the “Ligand Activation” theory, DSL proteins that have just been synthesized and reached the cell surface are still inactive and do not have the capacity to activate the Notch receptor on the signal receiving cell. DSL are endocytosed and sorted into a unique endocytic compartment where they become “activated”. The activated ligands return to the cell surface via the recycling pathway where they interact with and activate the Notch receptor. In contrast, the “pulling force” model insists that when DSL ligands and Notch receptor interact, endocytosis in the signal sending cell generates a mechanical force that leads to a conformational change in the Notch receptor. This force mediates the separation of the Notch heterodimer, and allows the S2 cleavage mediated by ADAM proteases. The extracellular portion of Notch is trans-endocytosed into the signal sending cell and assumed to be degraded through the lysosomal pathway along with the DSL ligands.