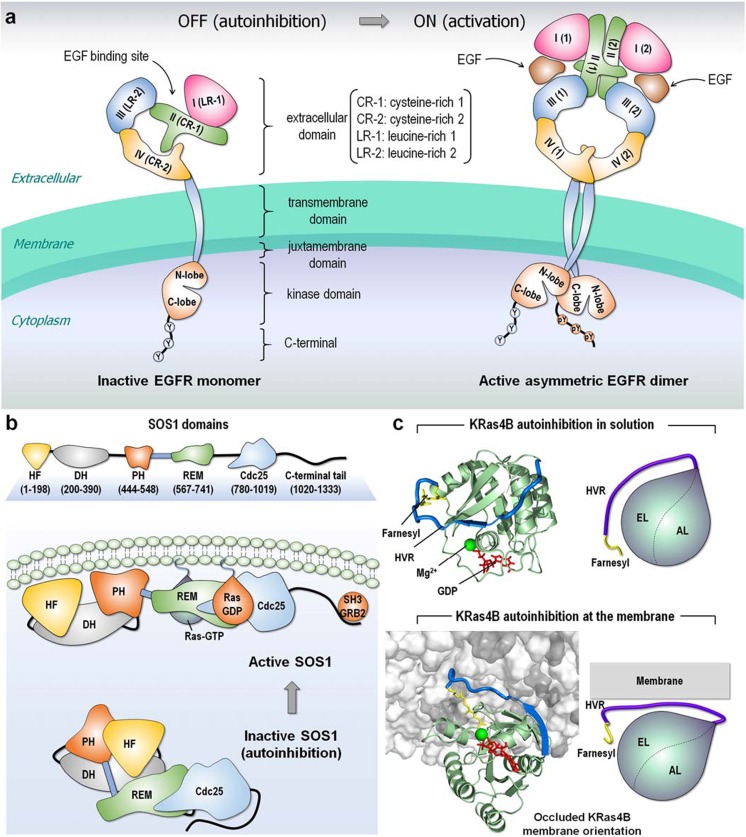
Fig. 2.
Examples of autoinhibition. a Collapsed conformation of the extracellular domain retains EGFR in an inactive, autoinhibition state. The inactive EGFR monomer can form a symmetric dimer but remains in the inactive state. The autoinhibition is released when the EGF ligand binds to the extracellular domain, causing an extended conformation of the extracellular domain and resulting in an asymmetric assembly of the kinase domains. This shifts the population to the active EGFR dimer. b SOS1, a GEF for Ras, contains histone-like fold (HF), Dbl-homology (DH), and pleckstrin-homology (PH) domains at the N-terminal region, and REM, Cdc25, and C-terminal SH3 binding motif at the C-terminal catalytic region. Inactive SOS1 is autoinhibited by the DH and PH domains that hamper the REM allosteric site for Ras binding. The C-terminal tail also blocks the Cdc25 catalytic site for Ras binding. The SH3 domain of growth factor receptor bound protein-2 (GRB2) binds the proline-rich SOS1 C-terminal tail and recruits SOS1 to the cell membrane. Membrane interactions of the DH and PH domains release autoinhibition and Ras association. c Autoinhibitions of KRas4B-GDP in solution and at the membrane interaction. In the inactive autoinhibition state, the HVR covers the effector binding site, resulting in occlusion of the catalytic domain membrane orientation. The KRas4B structures were adopted from previous work (Jang et al. 2016)