Abstract
Background
Anemia is a common hematologic disorder among human Immunodeficiency virus (HIV) infected adult Individuals. However, there is no concrete scientific evidence established at national level in Ethiopia. Hence, this review gave special emphasis on Ethiopian HIV infected adult individuals to estimate pooled prevalence of anemia and its associated factors at national level.
Methods
Studies were retrieved through search engines in PUBMED/Medline, Cochrane Library, and the web of science, Google and Google scholar following the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P). Joanna Briggs Institute Meta-Analysis of Statistical Assessment and Review Instrument (JBI-MAStARI) was used for critical appraisal of the included studies. Random effects meta-analysis was used to estimate the pooled prevalence of anemia and associated factors at 95% Confidence interval with its respective odds ratio (OR). Meta regression was also carried out to identify the factors. Moreover, Sub-group analysis, begs and egger test followed by trim-and-fill analysis were employed to assess heterogeneity and publication bias respectively.
Result
A total of 532 articles were identified through searching of which 20 studies were included in the final review with a total sample size of 8079 HIV infected adult individuals. The pooled prevalence of anemia was 31.00% (95% CI: 23.94, 38.02). Cluster of Differentiation 4 (CD4) count <= 200 cells/μl with OR = 3.01 (95% CI: 1.87, 4.84), World Health Organization (WHO) clinical stage III&IV with OR = 2.5 (95% CI: 1.29, 4.84), opportunistic infections (OIs) with OR = 1.76 (95% CI: 1.07, 2.89) and body mass index (BMI) < 18.5 kg/M2 with OR = 1.55 ((95% CI: 1. 28, 1.88) were the associated factors.
Conclusion
This review demonstrates high prevalence of anemia among HIV infected adults. Low CD4 count, WHO clinical stage III&IV, OIs and low level of BMI were found to have significant association with the occurrence of anemia. Therefore, the responsible stockholders including anti retro viral treatment (ART) clinics should strengthen the system and procedures for the early diagnosis of opportunistic infection and screening of underlying problems. There should be also early screening for OIs and under nutrition with strict and frequent monitoring of HIV infected individuals CD4 count.
Keywords: Anemia, Adult individuals, Pooled prevalence, Ethiopia, HIV/AIDS
Background
Anemia is occurs when the number of red blood cells or their oxygen carrying capacity becomes insufficient to meet the physiologic condition [1]. Anemia is also common hematologic disorder among HIV infected individuals [2] with impact on quality of life and clinical outcomes among these individuals [3].
This hematologic disorder may be attributed by HIV infection itself (the incidence is increased from asymptomatic to final Acquired Immuno Deficiency Syndrome (AIDS) stage) [4],OIs, gastrointestinal bleeding, nutritional deficiencies and erythropoietin depletion [5–7]. The complex interplay of all those factors cause life threatening symptoms, which also increases the hazard of mortality among HIV infected individuals [5, 8].
A compressive global estimate of anemia in the year from 1990 to 2010 was 32.9%, resulting in 68.4 million years lived with disability (YLD) [9]. Another systematic analysis of global anemia in the year 1990–2013 also reported the high burden of anemia in which developing countries account nearly 90 % of all anemia-related disability [10].
The prevalence of anemia among HIV infected adult individuals ranges from 20 to 80% and associated with high burden of morbidity and mortality [11]. Anemia among HIV infected individuals is a well-documented phenomenon and need to be the focus area of research to determine the prevalence and its predictors in a specific country [11, 12].
Based on this recommendation, a number of researches were done and reported that the prevalence of anemia among HIV infected adult individuals was:11.2% at northwest parts of Ethiopia in 2014 [3], 11.4% at Addis Ababa in 2018 [13],11.5% at northwest parts of Ethiopia in 2014 [14], 12% at southern parts of Ethiopia in 2005–2010 [15],14.3% at Addis Ababa in 2011/2012 [16], 22.6 at southern parts of Ethiopia in 2016 [17], 23%at northwest parts of Ethiopia in 2015 [18], 23.1% at Southwest parts of Ethiopia in 2012 [19], 25% at Northwest parts of Ethiopia in 2016 [20], 32.48% at Addis Ababa in 2011/2012 [21], 33% at Addis Ababa in 2008–2012 [22], 34.4% at northeast parts of Ethiopia in 2010–2013 [23], 35% at northwest Ethiopia in 2011/2012 [24], 41.2% at Eastern parts of Ethiopia in 2014 [25], 43% at northwest parts of Ethiopia in 2012/2013 [26], 51.74% at Gambella region in Southwest parts of Ethiopia in 2014 [27], 53.3% in Southern parts of Ethiopia in 2016–2013 [3] and 70.1% at northwest parts of Ethiopia in 2011/2012 [28].
The above individual studies revealed that the findings pertaining to the prevalence of anemia are inconsistent across regions and had variations over time. Similar to the prevalence of anemia, predictor variables were also inconsistent among the aforementioned studies. In addition to this gap, lack of documented data on pooled prevalence of anemia and its associated factors among HIV infected adult individuals at national level hinders program managers to design and implement effective strategies.
With the existing meager evidence in developing countries, we set out to explore the evidence on national pooled prevalence of anemia, and to our knowledge, found no published systematic review and meta-analysis so far on this topic to help guide decision-making. Therefore, this review would summarize the available evidence on the pooled prevalence of anemia among HIV infected adult individuals and associated factors.
Methods
Search strategy
Initially databases were searched for same systematic review and meta-analysis to avoid duplications. First DARE database (http://www.library.UCSF.edu) was explored in an attempt to confirm whether systematic review or meta-analysis exists and for availability of ongoing projects related to the topic. We also searched the three Trials Registries: ICTRP, Clinical Trials.gov and PROSPERO (searched January 2018). By using this method, it was confirmed that there was no any review and meta-analysis was conducted in similar to with this topic.
We systematically reviewed and analyzed published research articles to determine the pooled prevalence of anemia and its associated factors among HIV infected adult individuals in Ethiopia. To identify published articles, major databases such as PUBMED/MEDLINE, WHOLIS, Cochrane library, web of science, Google and Google Scholar were used accordingly. In addition, reference lists of relevant studies were identified and the full-text articles reviewed for inclusion. The key term used for PubMed search were “Prevalence” OR “incidence” AND “Anemia” AND “HIV” OR “AIDS” AND “Ethiopia”. The Search terms were pre-defined to allow a comprehensive search strategy that included all fields within records and Medical Subject Headings (MeSH terms) was used to help expand the search in advanced PubMed search. This study also used Boolean operator (within each axis we combined keywords with the “OR” operator and we then linked the search strategies for the two axes with the “AND” operator). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline during the systematic review [29].
Eligibility criteria
We reviewed abstracts from initial search using defined inclusion and exclusion criteria.
Inclusion criteria
Study scope
All studies which determine the prevalence of anemia and which identify the associated factors of it among HIV infected adult individuals were included under this systematic review and meta-analysis.
Study design
Both cross sectional and cohort study designs were included.
Study setting
Both community and health institution level studies.
Language
All articles published in English language were included.
Population
All HIV infected adult individuals in Ethiopia.
Publication and publication year
Published articles until May 05/2018 were included.
Exclusion criteria
Based on the eligibility criteria, we read their titles and abstracts. If studies are relevant for our review, we examined the full texts. Those papers which didn’t fully accessed at the time of our search process were excluded from this review after contact was attempted with the principal investigator through email at least two times. After reading the abstracts, if the studies are relevant to our review, we examined the full texts. The reason for the exclusion of these articles is that we are unable to assess the quality of each article in the absence of their full texts. Studies which didn’t report specific outcomes for anemia and associated factors quantitatively were also excluded from this systematic review and meta-analysis. Moreover, studies with poor quality as per settled criteria and review articles were also excluded from the review.
Data abstraction
The Database search results were combined and duplicate articles were removed manually using Endnote (version X8). Data were extracted by two authors using a standardized data extraction spread sheet. The data extraction spreadsheet was piloted on 7 randomly selected papers and modified accordingly. Data extraction sheet included study characteristics such as: (1) Authors’ name, year, region, study or publication year, study design, study setting and study population; (2) incidence or prevalence of anemia (3) data extracted on sex, OIs, CD4 counts, WHO clinical stages, residence, their nutritional status, residence and their history of malaria infestation; (4) studies’ quality score, sampling technique, study area, estimating technique of anemia using Hemoglobin measurement and their mean age of the study participants were also extracted from each individual studies.
Quality assessment (appraisal) of the individual studies
The Database search results were combined and duplicate articles were removed manually using Endnote (version X8). Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI) adapted for both cross sectional and follow up study design was used [30]. Three independent reviewers critically appraised each individual paper. Disagreements between those reviewers were solved by discussion. If not third reviewer was involved to resolve inconsistencies in between the two independent reviewers. Studies which score five and above were included in the final systematic review and meta-analysis.
Outcome measurements
This review has two main outcomes. The primary outcome was prevalence of anemia among HIV infected adult individuals which was calculated as number of adult HIV infected individuals who experienced anemia divided by total adult HIV infected individuals who were at risk of developing anemia and multiplied by 100%. The second aim of this review was to identify predictors of anemia among adult HIV infected individuals in Ethiopia.
Data analysis and synthesis
The extracted data were entered into computer using excel sheet and imported to STATA 14 for analysis. Evidence of publication bias was assessed using both egger’s test and begg’s test with p-value of less than 0.05 as a cut of point to declare the presence of publication bias [31, 32]. Heterogeneity across studies was checked using the inverse variance (I2) with Cochran Q statistic at 25, 50 and 75% as low, moderate and sever heterogeneity respectively [33]. Forest plot was also used to visualize the presence of heterogeneity. P value less than 0.05 was also used to declare the presence of heterogeneity across studies. Potential differences between studies were explored by sub-group analyses and Meta regression. The impact of heterogeneity across studies on the meta-analysis was quantified by I-square statistic (TAU) and a cutoff point of 50% was used to declare substantial heterogeneity. The effect size of categorical data was expressed using odds ratio.
Measures of effect and reporting
PRISMA flow diagrams used to summarize the study selection process. Random effects (DerSimonian and laird) model was used during analysis and Odds Ratios with their 95% CI were used to present the pooled effect sizes.
Results
Selection of studies
A total of 532 articles searched through the electronic (526) and supplementary (6) searches of which 78 duplicated articles were excluded. From the remaining 454 articles, 429 articles were excluded after reading of titles and abstracts based on the pre-defined inclusion criteria’s. Finally, 25 full text articles were accessed and assessed for eligibility criteria. Based on the pre-defined criteria and after critical appraisal, only 20 articles were included for the final analysis (Fig. 1).
Fig. 1.
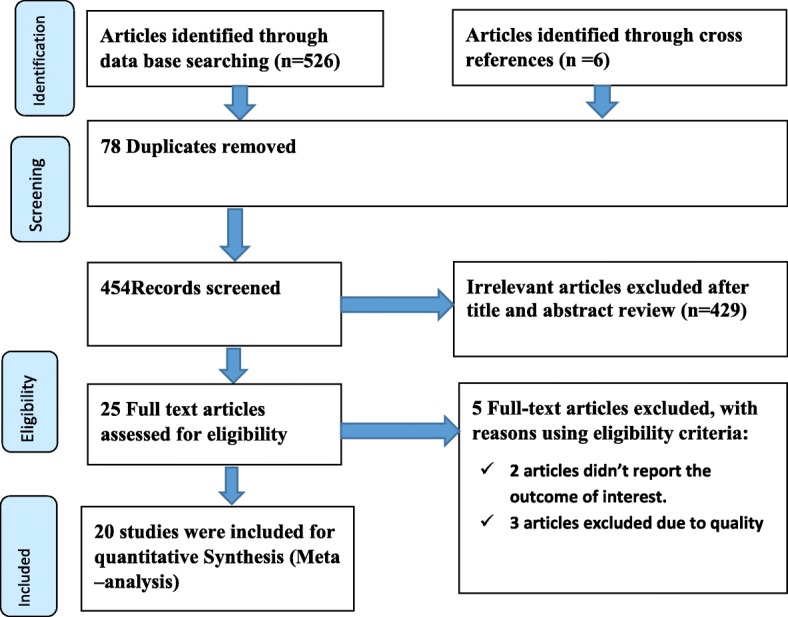
PRISMA flow diagram of included studies to estimate the pooled prevalence of anemia and its predictors among HIV infected adult individuals in Ethiopia from 2005 up to 2017
Characteristics of included studies
A total of twenty articles met the inclusion criteria. All the included studies were published between 2005 and 2017. Both cross sectional and cohort studies were included accordingly using an estimated sample size range from 172 [27] up to 1061 [16] adult HIV infected individual samples who were taken at Southwest parts of Ethiopia in the year 2014 and Addis Ababa city in the year 2011/2012 respectively. About 3139 men and 4920 women with a total sample of 8, 059 adult HIV infected individuals were included to estimate the pooled prevalence of anemia and its associated factors among adult HIV infected individuals (Table 1).
Table 1.
characteristics of included studies to estimate the pooled prevalence of anemia and its predictors among adult HIV infected individuals in Ethiopia from 2005 up to 2017
| ID | Region | Author | Study year | Publication year | Study design | Sampling method | Sample size | Gender distribution | Mean age In years | Measurement of anemia | Quality score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||||
| 1 | Addis Ababa | Tamir Z et al. [21] | 2011/2012 | 2018 | Cohort | _ | 394 | 142 | 252 | _ | Hemoglobin level | 7 |
| 2 | SNNP | Alamdo A et al. [3] | 2006–2012 | 2015 | Cross sectional | _ | 411 | 216 | 195 | 33.9 | Hemoglobin level | 6 |
| 3 | Addis Ababa | Woldeamanuel G et al. [13] | 2017 | 2018 | Cross sectional | Simple random | 255 | 148 | 107 | 40.6 | Hemoglobin level | 8 |
| 4 | Amhara | Beyene H et al. [26] | 2012/2013 | 2017 | Cross sectional | _ | 528 | 278 | 250 | 33.69 | Hemoglobin level | 8 |
| 5 | Amhara | Melese H et al. [18] | 2015 | 2017 | Cross sectional | Simple random | 377 | 234 | 143 | 35.21 | Hemoglobin level | 8 |
| 6 | Amhara | Alem M et al. [28] | 2011/2012 | 2013 | Cross sectional | Simple random | 384 | 233 | 151 | 37 | Hemoglobin level | 8 |
| 7 | Amhara | Deressa et al. [20] | 2016 | 2018 | Cross sectional | Systematic | 320 | 203 | 117 | _ | Hemoglobin level | 6 |
| 8 | Amhara | Fiseha T et al. [23] | 2010–2013 | 2013 | Cohort | Simple random | 373 | 233 | 140 | 34.6 | Hemoglobin level | 8 |
| 9 | Amhara | Tesfaye Z et al. [14] | 2014 | 2014 | Cohort | _ | 349 | 218 | 131 | 34.6 | Hemoglobin level | 8 |
| 10 | Oromia | Gedefaw L et al. [19] | 2012 | 2013 | Cross sectional | _ | 234 | 145 | 89 | 32.09 | Hemoglobin level | 6 |
| 11 | Addis Ababa | Wolde H et al. [22] | 2008–2012 | 2014 | Cohort | Simple random | 616 | 401 | 215 | _ | Hemoglobin level | 5 |
| 12 | Addis Ababa | Assefa M et al. [16] | 2011/2012 | 2015 | Cohort | Simple random | 1061 | 632 | 429 | _ | Hemoglobin level | 8 |
| 13 | Amhara | Bamlaku E et al. [2] | 2012/2013 | 2014 | Cohort | _ | 319 | 202 | 117 | _ | Hemoglobin level | 5 |
| 14 | SNNP | Daka D et al. [15] | 2005–2010 | 2013 | Cross sectional | _ | 384 | 254 | 130 | 33.64 | Hemoglobin level | 7 |
| 15 | Amhara | Ferede G et al. [24] | 2011/2012 | 2013 | Cohort | _ | 400 | 278 | 122 | _ | Hemoglobin level | 5 |
| 16 | Dire Dawa | Geleta D et al. [25] | 2014 | 2016 | Cross sectional | Simple random | 425 | 275 | 150 | 34 | Hemoglobin level | 8 |
| 17 | Gambella | Sahle T et al. [27] | 2014 | 2017 | Cross sectional | Systematic | 172 | 68 | 104 | 31.95 | Hemoglobin level | 8 |
| 18 | SNNP | Muluken W et al. [17] | 2016 | 2018 | Cross sectional | Simple random | 376 | 195 | 181 | _ | Hemoglobin level | 5 |
| 19 | SNNP | Gedel G et al. (2015) [34] | 2013/2014 | 2015 | Cross sectional | Systematic | 305 | 189 | 116 | 39.5 | Hemoglobin level | 8 |
| 20 | Tigray | Hadgu T et al. (2013) [35] | 2012 | 2013 | Cross sectional | Systematic | 376 | 376 | _ | 32.5 | Hemoglobin level | 8 |
Of the total 20 articles, more than one third of studies were conducted in Amhara regional state health institutions [2, 14, 18, 20, 23, 24, 26, 28]; four studies in Addis Ababa health institutions [13, 16, 21, 22]; four studies at South Nations Nationalities and peoples of Ethiopia national regional state (SNNP) [3, 15, 17, 34]; one study at Oromia national regional state [19], one study at Gambella national regional state [27], one study at Tigray regional national state [35] and one study at Dire Dawa town administration of Ethiopia [25].
Regarding the study designs, 13 studies [3, 13, 15, 17–20, 25–28, 34, 35] and the remaining seven were cross sectional and cohort studies respectively. After critical appraisal of each articles based on JBI-MAStARI, both in peer and independently, they scored in the range of 5-up to 8 out of 9 values (see Table 1).
Prevalence of anemia among adult HIV infected individuals in Ethiopia (Meta-analysis)
As shown in the forest plot below, the pooled prevalence of anemia among adult HIV infected individuals in Ethiopia was 31.00% (95% CI: 23.94, 38.02) (Fig. 2). Substantial level of statistically significant heterogeneity was detected (I2 = 98%; p < 0.001) suggesting that the use of random effects model in estimating the pooled estimates is appropriate. The substantial magnitude of the heterogeneity also suggests the need to conduct subgroup analysis which demands identifying the sources of heterogeneity (Fig 2).
Fig. 2.

Forest plot showing the pooled prevalence of anemia among adult HIV infected individuals in Ethiopia from2005 up to 2017
Subgroup analysis
Since this review is exhibited with substantial heterogeneity, subgroup analysis based on study year, type of study design, study year, mean age in years, gender distribution, sample size and sampling technique they used were considered to identify the possible source of heterogeneity across studies (Table 2). However the subgroup analysis result indicated that the source of heterogeneity was not due to type of study design, study year, mean age in years, gender distribution, sample size and sampling technique they used.
Table 2.
Sub group analysis which describes pooled prevalence of anemia and its predictors among adult HIV infected individuals in Ethiopia
| Sub group | Number of included studies | Prevalence (95% CI) | Heterogeneity statistics | P value | I2 | |
|---|---|---|---|---|---|---|
| By study design | Cross sectional | 13 | 34.54 (24.54, 44.55) | 761.77 | < 0.001 | 98.4 |
| Cohort | 7 | 24.44 (16.18, 32.70) | 228.57 | < 0.001 | 97.4 | |
| Time of study year | Before 2015 | 16 | 33.65 (25.13, 42.16) | 1077.02 | < 0.001 | 98.6 |
| 2015 and above | 4 | 20.43 (14.12, 26.74) | 26.29 | < 0.001 | 88.6 | |
| Gender distribution | Male | 12 | 41.21 (27.07,55.36) | 774.85 | < 0.001 | 98.6 |
| Female | 12 | 34.52(24.27,44.77) | 309.79 | < 0.001 | 96.4 | |
| sampling method | Simple random | 8 | 31.20 (18.86, 43.54) | 598.36 | < 0.001 | 98.8 |
| Systematic | 4 | 37.37 (23.72, 51.02) | 77.69 | < 0.001 | 96.1 | |
| Un-defined | 8 | 27.64 (16.62, 38.66) | 413.30 | < 0.001 | 98.3 | |
| Mean age in years | <= 35 | 8 | 34.31 (21.75, 46.88) | 432.49 | < 0.001 | 98.4 |
| > 35 | 5 | 34.54 (14.59, 54.49) | 422.51 | < 0.001 | 99.1 | |
| No report of mean age | 7 | 24.71(17.28, 32.14) | 172.93 | < 0.001 | 96.5 | |
| Sample size | < 384 | 11 | 25.91 (18.63, 33.19) | 296.43 | < 0.001 | 98.3 |
| > = 384 | 9 | 37.1 (24.68,49.51) | 1141.04 | < 0.001 | 99.0 | |
Publication bias was observed using both begg’s and egger’s test [36, 37] and the value was found to be significant at p value of 0.002 and 0.004 respectively. So that trim and fill meta-analysis [38] has been done to account for the publication bias. Based on this analysis, the prevalence of anemia among HIV infected individuals was 31% (95% CI: 23.97, 38.02) and no significant change was exhibited as compared with the main meta-analysis.
Meta regression
In addition to subgroup analysis and publication bias, Meta regression was also undertaken by considering both continuous and categorical data to identify associated factors with the pooled prevalence of anemia. Sample size, study year, publication year, study design, Gender distribution, sampling technique and mean age in years for each individual studies were considered in the meta-regression. But the meta regression showed that the pooled prevalence of anemia among HIV infected adult individuals was not associated with sample size, study year, publication year, study design, Gender distribution, sampling technique and mean age in years (Table 3).
Table 3.
Meta regression for the included studies to identify source of heterogeneity for the prevalence of anemia among HIV infected adult individuals in Ethiopia from 2005 up to 2017
| Variables | Characteristics | Coefficients | P-value |
|---|---|---|---|
| Year | Publication year | −.8434435 | 0.672 |
| Study year | Before 2015 | Reference | Reference |
| 2015 and above | −13.14142 | 0.156 | |
| Sample | Sample size of each articles | −.0092616 | 0.665 |
| Study design | Cohort | −10.0263 | 0.199 |
| Cross sectional | Reference | Reference | |
| Gender distribution | Male | .0071367 | 0.883 |
| Female | Reference | Reference | |
| Sampling technique | Simple random | 3.11105 | 0.747 |
| Systematic | 15.15263 | 0.385 | |
| Undefined | Reference | Reference | |
| Mean age in years | <=35 | 9.531214 | 0.282 |
| > 35 | 9.762604 | 0.328 | |
| No report of mean age | Reference | Reference |
Associated factors of anemia among HIV infected adult individuals in Ethiopia
Low CD4 count, WHO clinical stage III&IV, OIs and low level of BMI were found to have significant association with the occurrence of anemia among HIV infected adult Individuals.
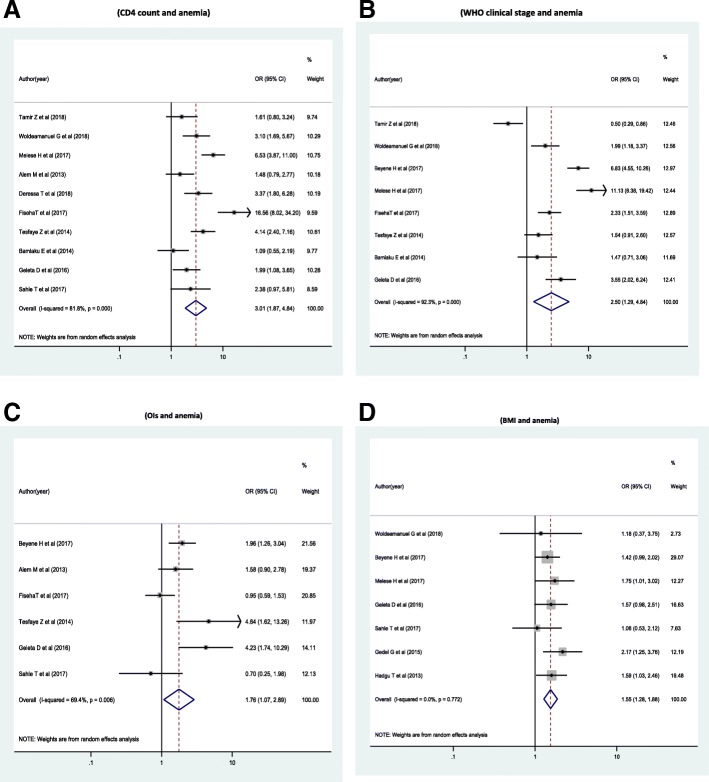
The odds of developing anemia were 3 times higher among HIV infected individuals with CD4 count of < 200 cell/ul compared with HIV infected individuals with CD4 count is > = 200/ul [(OR = 3.01 (95% CI: 1.87, 4.84))] (Fig. 3a). The odds of developing anemia were 2.5 times higher among HIV infected Individuals with WHO clinical stage III &IV compared with individuals with WHO clinical stage I&II [OR = 2.5 (95% CI: 1.29, 4.84)] (Fig. 3b). The odds of developing anemia were 1.8 times higher among HIV infected Individuals with opportunistic infection had compared with individuals free of opportunistic infections [OR = 1.76 (95% CI: 1.07, 2.89)] (Fig. 3c). The odds of developing anemia were 1.6 times higher among HIV infected Individuals whose body mass index (BMI) < 18.5 kg/m2 had compared with those whose BMI was > = 18.5 kg/M2[OR = 1.55 ((95% CI: 1. 28, 1.88)](Fig. 3d).
Fig. 3.
Forest plots which describe associated factors of anemia among HIV infected adult individuals in Ethiopia. a Forest plot which describe the association between anemia and CD4 count response among HIV infected adult individuals in Ethiopia. b Forest plot which describe the association between anemia and WHO clinical stage among HIV infected adult individuals in Ethiopia. c Forest plot which describe the association between anemia and OIs among HIV infected adult individuals in Ethiopia. d Forest plot which describe the association between anemia and BMI among HIV infected adult individuals in Ethiopia
Discussion
This systematic review and meta-analysis was established out to estimate the pooled prevalence of anemia and its associated factors among HIV infected adult individuals in Ethiopia from 2005 [15] up 2017 [13].
According to this review, one third of HIV infected adult individuals were found to be anemic in Ethiopia. The prevalence of anemia in the current systematic review is higher than the finding of the study conducted in Tanzania [39] which estimated only severe form of anemia. The finding is also lower than the finding of the study conducted in European countries [40] and south India [41].
This variation may be due to variation in hemoglobin cut off point to define anemia across those regions. In Ethiopian studies anemia was defined if hemoglobin level was less than 11 g/dl for both men and women according to WHO definition criteria for anemia. However, in the studies conducted in European countries anemia was considered as less than or equal to 14 g/dl for men and less than or equal to 12 g/dl for women. On the other hand, anemia was considered as less than 13 g/dl for men as and less than 12 g/dl for women in the South Indian stud.
Another explanation for the difference in magnitude may be attributed to the geographic location variation where altitude difference across Ethiopia, Tanzania and European countries may change the level of hemoglobin concentration. The meta analysis results also indicated that CD4 count of HIV infected individual was found to have statistically significant association with anemia. Decrease in CD4 count of HIV infected individual leads to the increase progression to [42]. This finding is consistent with the findings reported in the study conducted in the Kathmandu [43], the united states [4], in south Africa [44], in Tanzania [39], in China [45] and Central region of Ghana [46]. This is due to patients with low Cd4 count may exposed with different opportunistic infections that in turn exposed to anemia. Hence rate of falling hemoglobin level also indicates falling of CD4 count, which is a gold standard for monitoring HIV progression [47].
The finding of current review is also consistent with the findings of studies conducted in South Africa [44], south India [41], Tanzania [39] in which WHO clinical stage III/IV of HIV, low level of body mass index (< 18.5 kg/m2) and different forms of opportunistic infections were an independent predictors of anemia among HIV infected individuals.
In addition, evidences also documented that individuals with advanced stage of HIV exposed to OIs [48]. These OIs also may cause dietary problems which would led to nutritional deficiencies and problems of absorbing nutrients which in turn would lead to anemia and malnutrition [49].
Theoretical and practical implications
HIV infected adult individuals are more prone to develop concomitant infections demanding comprehensive package of health services including ART. This review evidence also indicated that those anemic HIV infected individuals deserve more attention where integrated service provision is critical to identify and manage underlying causes for anemia at early stages. Hence, specific considerations tailored to anemia screening and management needs to be taken into account during ART program design and implementation across the health system. Despite the vast investment of resources in tackling the occurrence of anemia in low and middle income countries few studies, are available to inform policy and decision making. Therefore, further studies are also recommended to generate pooled evidence at global level in general and in developing countries in particular.
Strengths and limitations
The main strength of the current review lies in our adherence to international standardized guidelines on the conduct and reporting of systematic reviews. We included studies only from peer-reviewed English-language journals, which may have restricted our findings. Though searching was done for unpublished papers, only published studies were included.
Conclusion
This systematic review and meta-analysis showed a high prevalence of anemia among HIV infected adult individuals in Ethiopian in which in 1/3 of HIV infected adult individuals were anemic. The review found that low CD4 count; advanced stage of the disease (WHO clinical stage III &IV), opportunistic infection and low level of BMI were found to have positive significant association with the development of anemia. Therefore, the responsible stockholders including ART clinics should strengthen the system and procedures for the early diagnosis of opportunistic infection and screening of underlying problems like under nutrition. The system should focus on technical update training on opportunistic infection diagnosis and treatment guidelines, on nutritional assessment, counseling and support (NACs), strengthening of laboratory facilities which can detect opportunistic infections early in infection. Moreover linkage of case managers, mother groups and HIV/AIDs union with ART clinic should also be strengthened for better referral linkage which can indirectly help for early screening of opportunistic infection and undernutrition. There should also be strict and frequent monitoring of HIV infected individuals CD4 count than previous in order to minimize anemia and related burdens.
Acknowledgments
We would like to thank all authors of studies included in this systematic review and meta-analysis.
Funding
Not applicable.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BMI
Body mass index
- CD4
Cluster of differentiation 4
- CI
Confidence Interval
- HIV
Human immuno virus
- OIs
Opportunistic Infections
- SNNP
South Nations Nationalities and peoples of Ethiopia
Authors’ contributions
AN and TG developed the protocol and involved in the design, selection of study, data extraction, statistical analysis and developing the initial drafts of the manuscript. ZA, TT, DJ and HT involved in quality assessment. AN, TG and DJ prepared and revising subsequent drafts. AN, ZA and TT prepared the final draft of the manuscript. All authors read and approved the final draft of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayenew Negesse, Email: ayenewnegesse@gmail.com.
Temesgen Getaneh, Email: temugetaneh@gmail.com.
Habtamu Temesgen, Email: habtamutem@gmail.com.
Tesfahun Taddege, Email: ttaddege@gmail.com.
Dube Jara, Email: jaradube@gmail.com.
Zeleke Abebaw, Email: zelekeabebaw7@gmail.com.
References
- 1.World Health Orgnanization: health topics on Anemia which is available at http://www.who.int/topics/anaemia/en/.
- 2.Enawgaw B, Tadele A, Melku M, et al. Prevalence of zidovudine induced megaloblastic anemia among human immunodeficiency virus positive patients attending University of Gondar hospital antiretroviral therapy clinic, Northwest Ethiopia. HIV Advance Res Dev. 2014;1(1):105. [Google Scholar]
- 3.Gizaw AA, Temesgen F, Amanuel T, et al. Anemia and its associated risk factors at the time of antiretroviral therapy initiation in public health facilities of Arba Minch Town, Southern Ethiopia. Health. 2015;7(12):1657. doi: 10.4236/health.2015.712179. [DOI] [Google Scholar]
- 4.Sullivan Patrick S, Hanson Debra L, Chu Susan Y. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91(1):301–308. [PubMed] [Google Scholar]
- 5.Sudhir M, Srinivasa J, Dinesh G. Hematologic manifestations of HIV/AIDS. Medicine. 2011;9:484–490. [Google Scholar]
- 6.Richman Douglas D, Fischl Margaret A, Grieco MH. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 7.Camacho J, Poveda F, Zamorano AF, et al. Serum erythropoietin levels in anaemic patients with advanced human immunodeficiency virus infection. Br J Haematol. 1992;82(3):608–614. doi: 10.1111/j.1365-2141.1992.tb06475.x. [DOI] [PubMed] [Google Scholar]
- 8.Volberding Paul A, Bake Kelty R, Levine Alexandra M. Human immunodeficiency virus hematology. ASH Educ Program Book. 2003;2003(1):294–313. doi: 10.1182/asheducation-2003.1.294. [DOI] [PubMed] [Google Scholar]
- 9.Kassebaum Nicholas J, Rashmi J, Mohsen N, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassebaum Nicholas J. The Global Burden of Anemia. Hematology/Oncology Clinics of North America. 2016;30(2):247–308. doi: 10.1016/j.hoc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Belperio Pamela S, Rhew David C. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(7):27–43. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Rawat Rahul MCSI, Suneetha AK. Poor diet quality is associated with low CD4 count and anemia and predicts mortality among antiretroviral therapy–naive HIV-positive adults in Uganda. J. Acquir. Immune Defic. Syndr. 2013;62(2):246–253. doi: 10.1097/QAI.0b013e3182797363. [DOI] [PubMed] [Google Scholar]
- 13.Garedew WG, Haile WD. Prevalence of anemia before and after initiation of antiretroviral therapy among HIV infected patients at black lion specialized hospital, Addis Ababa, Ethiopia: a cross sectional study. BMC Hematology. 2018;18(1):7. doi: 10.1186/s12878-018-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelalem T, Bamlaku E. Prevalence of anemia before and after initiation of highly active antiretroviral therapy among HIV positive patients in Northwest Ethiopia: a retrospective study. BMC. Res. Notes. 2014;7(1):745. doi: 10.1186/1756-0500-7-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deresse D, Dereje L, Aderajew A, et al. Prevalence of anaemia before and after the initiation of antiretroviral therapy at ART centre of Hawassa University Referral Hospital, Hawassa, South Ethiopia. Sch J Med. 2013;3(1):1–6. [Google Scholar]
- 16.Muluken A, Erku AW, Aster S, et al. Prevalence and correlates of anemia among HIV infected patients on highly active anti-retroviral therapy at Zewditu memorial hospital, Ethiopia. BMC Hematology. 2015;15(1):6. doi: 10.1186/s12878-015-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wubetu M, Mebratu E. Assessment of the prevalence of zidovudine induced Anemia among adult HIV/AIDS patients on HAART in an Ethiopian Hospita. Occupational Medicine & Health Affairs. 2018;6:271. doi: 10.4172/2329-6879.1000271. [DOI] [Google Scholar]
- 18.Hermela M, Mesele WM, Haile W, et al. Anemia among adult HIV patients in Ethiopia: a hospital-based cross-sectional study. HIV/AIDS (Auckland, NZ) 2017;9:25. doi: 10.2147/HIV.S121021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lealem G, Tilahun Y, Zewdineh S, et al. Anemia and risk factors in HAART naive and HAART experienced HIV positive persons in south West Ethiopia: a comparative study. PLoS One. 2013;8(8):e72202. doi: 10.1371/journal.pone.0072202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tekalign D, Debasu D, Meseret W, et al. Anemia and thrombocytopenia in the cohort of HIV-infected adults in Northwest Ethiopia: a facility-based cross-sectional study. EJIFCC. 2018;29(1):36. [PMC free article] [PubMed] [Google Scholar]
- 21.Zemenu T, Jemal A, Aster T. Anemia among HIV infected individuals taking art with and without zidovudine at Addis Ababa, Ethiopia. Ethiopian J Health Sci. 2018;28(1):73–82. doi: 10.4314/ejhs.v28i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milkias WH, Lerebo WT, Melaku YA, et al. Incidence and risk factors of Anemia among HIV/AIDS patients taking anti-retroviral erapy at tertiary hospitals in Addis Ababa, Ethiopia: a retrospective cohort study. J HIV AIDS Infect Dis2. 2014:1–06.
- 23.Temesgen F, Zemenu T, Abdurahaman S, et al. Prevalence of anemia in renal insufficiency among HIV infected patients initiating ART at a hospital in Northeast Ethiopia. BMC Hematology. 2017;17(1):1. doi: 10.1186/s12878-017-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getachew F, Yitayih W. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University hospital, Northwest Ethiopia. BMC Blood Disorders. 2013;13(1):8. doi: 10.1186/2052-1839-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dessalegn G, Bayissa DD, Birhanu S, et al. Prevalence of Anemia and associated factors among PHIVs attendants antiretroviral therapy clinics in public health institutions in Dire Dawa town, East Ethiopia. J Med, Physiol and Biophys. 2016;22.
- 26.Bedimo BH, Mulualem T, Disass H. Concurrent Plasmodium infection, anemia and their correlates among newly diagnosed people living with HIV/AIDS in northern Ethiopia. Acta Trop. 2017;169:8–13. doi: 10.1016/j.actatropica.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Tsion S, Tilahun Y, Lealem G. Effect of malaria infection on hematological profiles of people living with human immunodeficiency virus in Gambella, southwest Ethiopia. BMC Hematology. 2017;17(1):2. doi: 10.1186/s12878-017-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meseret A, Tigist K, Negash B, et al. Prevalence of anemia and associated risk factors among adult HIV patients at the anti-retroviral therapy clinic at the University of Gondar Hospital, Gondar, Northwest Ethiopia. Scientific Reports. 2013;2:article 3. [Google Scholar]
- 29.David M, Alessandro L, Jennifer T, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. [PubMed] [Google Scholar]
- 30.Institute Jonna Briggs . Meta-analysis of statistics: assessment and review instrument (JBI Mastari) Adelaide: Joanna Briggs Institute; 2006. p. 20032. [Google Scholar]
- 31.Begg Colin B., Mazumdar Madhuchhanda. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50(4):1088. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 32.Matthias E, Davey SG, Martin S, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins Julian PT, Thompson Simon G, Deeks Jonathan J, et al. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dereje G, Baye G, Dagnachew M, et al. Prevalence of malnutrition and its associated factors among adult people living with HIV/AIDS receiving anti-retroviral therapy at Butajira Hospital, Southern Ethiopia. BMC Nutrition. 2015;1(1):5. doi: 10.1186/2055-0928-1-5. [DOI] [Google Scholar]
- 35.Hailu HT, Walelegn W, Desalegn T, et al. Undernutrition among HIV positive women in Humera hospital, Tigray, Ethiopia, 2013: antiretroviral therapy alone is not enough, cross sectional study. BMC Public Health. 2013;13(1):943. doi: 10.1186/1471-2458-13-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begg Colin B., Mazumdar Madhuchhanda. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50(4):1088. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duval S, aT R. Trim and fll: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 39.Abel M, James O, Donna S, et al. Burden and determinants of severe anemia among HIV-infected adults: results from a large urban HIV program in Tanzania, East Africa. J Int Assoc Providers of AIDS Care (JIAPAC) 2015;14(2):148–155. doi: 10.1177/2325957413488195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amanda M, Ole K, Barton Simon E, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. Aids. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 41.Ramnath S. Anemia among HIV-infected individuals in South India. 2007. [Google Scholar]
- 42.Spivak Jerry L. Serum Immunoreactive erythropoietin. Jama. 1989;261:3104–3107. doi: 10.1001/jama.1989.03420210052015. [DOI] [PubMed] [Google Scholar]
- 43.Raj BK, Surya D, Midhan S, et al. Profile of Anaemia in HIV positive patients. J College of Med Sci-Nepal. 2016;12(2):70–73. doi: 10.3126/jcmsn.v12i2.15488. [DOI] [Google Scholar]
- 44.Simbarashe T, Mhairi M, Brennan Alana T, et al. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Tropical Medicine. 2013;2013:6. [DOI] [PMC free article] [PubMed]
- 45.Yinzhong S, Zhenyan W, Hongzhou L, et al. Prevalence of anemia among adults with newly diagnosed HIV/AIDS in China. PLoS One. 2013;8(9):e73807. doi: 10.1371/journal.pone.0073807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christian O, Yeboah Francis A, et al. Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited setting. J Biomed Sci. 2009;16(1):102. doi: 10.1186/1423-0127-16-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langford Simone E, Jintanat A, Cooper David A. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4(1):11. doi: 10.1186/1742-6405-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Center for Disease prevention and control (CDC): Opportunistic infection among HIV infected indviduals last updated may 30/2017: available at https://www.cdc.gov/hiv/basics/livingwithhiv/opportunisticinfections.html.
- 49.Porter C. A report on HIV/AIDS and malnutrition locked in vicious cycle. Washington File, 16 October 2006: available at http://aids.immunodefence.com/2006/10/hivaids-and-malnutrition-locke.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.