Abstract
In this review, we analyze the epidemiology of thromboses related to end-stage liver disease (ESLD), discuss causes of hypercoagulability, describe susceptible populations, and critically evaluate proposed prophylaxis and treatment of thromboses. Classically, ESLD has been regarded as a model for coagulopathy, and patients were deemed to be at high risk for bleeding complications. Patients with ESLD are not auto-anticoagulated, and they do not have a lower risk of portal vein thrombosis, intracardiac thrombus formation, pulmonary embolism or hepatic artery thrombosis. Though the cause of hypercoagulability is multifactorial, endothelial dysfunction likely plays a central role for all patients with ESLD. Some subpopulations, such as patients with nonalcoholic steatohepatitis and autoimmune conditions, are at increased risk of thrombotic events as are patients of Hispanic ethnicity. The science behind prophylaxis of different types of clotting and treatment of thromboses is developing rapidly. A number of medications, including low molecular weight heparin, unfractionated heparin, aspirin, vitamin K antagonists, and direct oral anticoagulants can be used, but clear guidelines are lacking. Acute intraoperative clotting can be associated with high mortality. Routine use of transesophageal echocardiography can be helpful in early recognition and treatment of intraoperative thrombosis. Heparin should be reserved for cases of intracardiac thrombus/pulmonary embolism without hemodynamic instability. In unstable patients, low dose of recombinant tissue plasminogen activator can be used. In this new era of heightened awareness of thrombotic events in ESLD patients, prospective randomized trials are urgently needed to best guide clinical practice.
End-stage liver disease (ESLD) is associated with complex alterations in the physiology of multiple biological systems in the human body. One of these fundamental changes is the reduced ability of the liver to synthesize proteins, including procoagulation and anticoagulation factors. Classically, ESLD has been regarded as a model for coagulopathy, and patients were deemed to be at high risk for bleeding complications. It has, however, been demonstrated that patients with cirrhosis rarely have unprovoked bleeding when compared to patients on anticoagulant therapy, patients with other acquired coagulopathies, or those with congenital coagulation deficiencies.1 If bleeding does occur, the sites are typically gastrointestinal and related to increased portal pressure.
Early liver transplantations (LT) were associated with dramatic bleeding and required massive transfusions of fresh frozen plasma and other blood products to correct abnormal hemostasis, as reflected by laboratory tests. Over time, due to improved surgical procedures and anesthesia management, the need for transfusions has declined,2 arguing against the clinical relevance of standard laboratory assays, such as the International Normalized Ratio (INR), activated partial thromboplastin time, and blood platelet count in assessment and prediction of bleeding tendency.3 Research has clearly demonstrated that patients with ESLD are not auto-anticoagulated, but predisposed to thromboses during each stage of LT.4
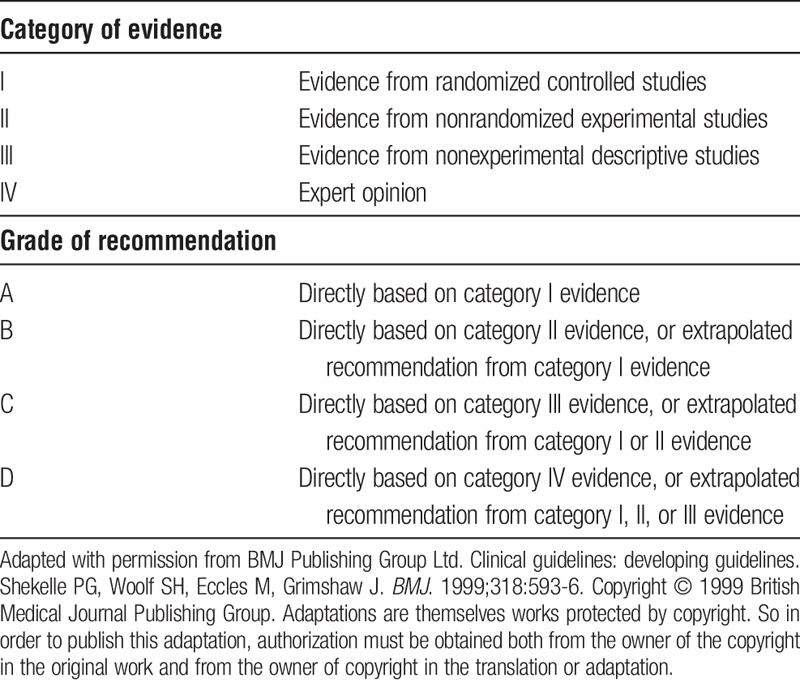
Recently, much has been published on hypercoagulability in ESLD. This review provides a broad overview of this subject. Based on most recent important literature, the authors also made recommendations for prophylaxis and treatment of thromboses, with emphasis on the period preceding and immediately after LT. Determination of levels of evidence and recommendation for treatment were based on current guidelines.5
CAUSES OF HYPERCOAGULABILITY IN ESLD
Hypercoagulability in patients with ESLD is associated with fundamental changes in the coagulation profile at the level of primary, secondary and tertiary hemostasis. Though the cause of hypercoagulability is multifactorial, endothelial dysfunction (ED) likely plays a central role.
The endothelium is the largest organ in the human body, comprising of more than 1013 cells.6 It separates blood from underlying tissue thereby maintaining coagulation homeostasis at the vascular wall. Endothelial dysfunction alters vascular tone as well as local pro/anticoagulant balance due to expression of tissue factor on the surface of endothelial cells.7 Endothelial cells synthesize tissue factor pathway inhibitor which may be decreased in ESLD.8 Inflammation and oxidative stress also cause ED.9 Hepatic ED induced by inflammation is mediated by activation of Toll-like receptors and secretion of tumor necrosis factor-α.6 Endotoxin, also known as lipopolysaccharide, is a component of the cell wall of gram negative bacteria. Lipopolysaccharide is recognized by Toll-like receptors and induces production of tumor necrosis factor-α by monocytes in cirrhotic patients.10 Portal endotoxemia occurs under physiological conditions.11 Endotoxin is absorbed from the colon and cleared by hepatic Kupffer cells. Liver injury leads to leakage of endotoxin into the systemic circulation due to reduced reticuloendothelial clearance and portosystemic shunts.7 Violi et al7 demonstrated elevated blood endotoxin concentrations in cirrhotic patients. In these patients, a gradient exists between endotoxin concentrations in the portal circulation and lower concentrations in the systemic circulation, albeit still higher than concentrations in controls. The same study demonstrated a correlation between endotoxemia and prothrombin F1 + 2, a marker of thrombin generation, suggesting an ongoing prothrombotic state in the portal circulation of cirrhotic patients. Nitric oxide dysregulation, a frequent finding in patients with ESLD, is associated with ED.12
ED is responsible for increased production of liver-independent coagulation factors such as von Willebrand factor (vWF), factor VIII (FVIII), and plasminogen activator inhibitor one (PAI-1).13-16 These alterations, in combination with changes in the balance of coagulation/anticoagulation and fibrinolytic/antifibrinolytic factors, affect all levels of the hemostatic system (Figure 1).
FIGURE 1.
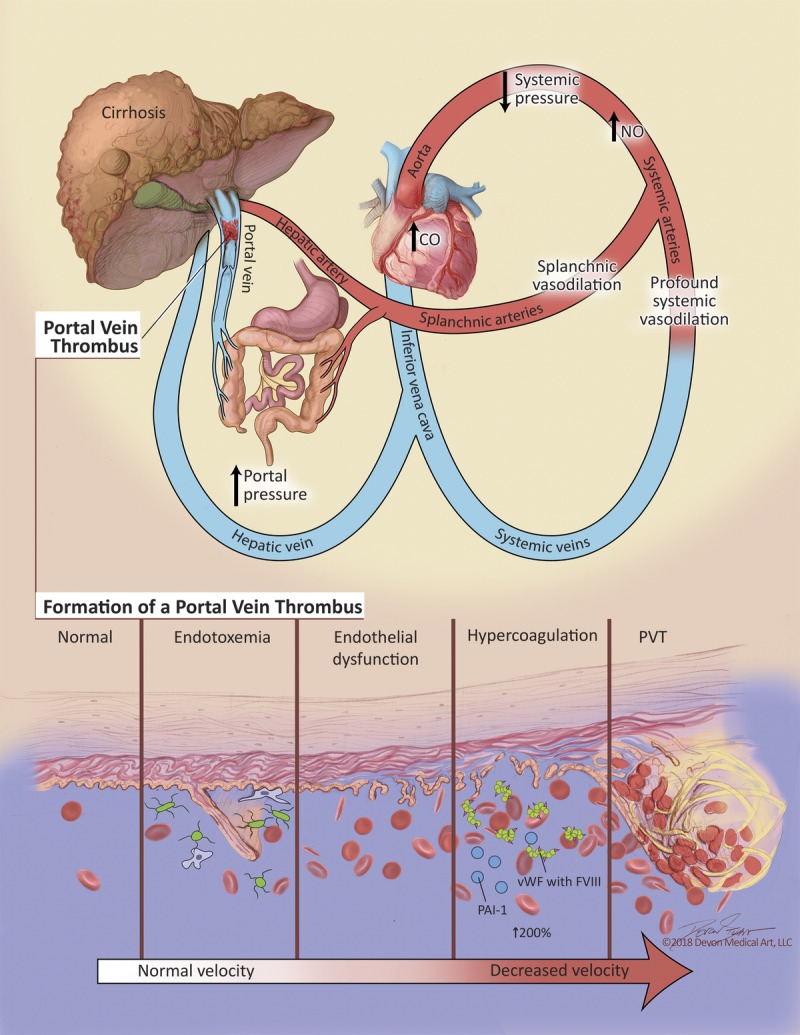
Summary of factors contributing to portal vein thrombosis.
Cirrhotic patients are often thrombocytopenic due to splenic sequestration and also impaired.16 In addition, in vitro platelet function is reduced.17 Levels of vWF are abnormally high in ESLD and correlate with severity of liver disease. The cleaving protease ADAMTS13 regulates the multimeric structure of vWF. Cirrhotic patients have reduced ADAMTS13 levels with elevated amounts of high-molecular-weight vWF in circulation.18 Lisman et al16 found the functional capacity of vWF was reduced and ADAMTS13 levels were highly variable, concluding that high levels of vWF compensate for a reduction in function of the protein. Despite a reduced number of platelets due to splenic sequestration, this change in concentration of vWF is a key factor responsible for hypercoagulability. In addition, cirrhosis is associated with chronic inflammation and low-grade endotoxemia which may lead to platelet hyperreactivity suggesting that there is less of a primary hemostatic clotting defect present in cirrhotic patients than previously thought.19
Secondary hemostasis depends on coagulation factors, many of which are synthesized by the liver. Factor V and Vitamin K-dependent factors (II, VII, IX and X) are reduced in severe liver disease, reflected by an elevated INR.13 Proteins S and C are also vitamin K-dependent anticoagulants produced by the liver. Their concentrations are decreased in ESLD,20 thereby compensating for the reduction in plasma procoagulant levels.15 It has been demonstrated that in ESLD, not just the concentration, but also the activity of anticoagulation factors including proteins S, C as well as antithrombin III (ATIII) is significantly reduced.20 Conventional coagulation tests such as the INR do not allow full activation of protein C and do not reflect this compensation; therefore INR does not predict bleeding risk.1,20 Decreased concentration of protein C is also responsible for hypercoagulability due to its involvement in the thrombomodulin pathway. Under normal conditions thrombomodulin (an endothelial receptor) activates protein C with subsequent suppression of thrombin generation. In vitro thrombomodulin resistance increases with severity of liver disease suggesting a procoagulant imbalance.3 Resistance to thrombomodulin due to ED in combination with protein C deficiency can lead to thrombin overproduction. These changes, as well as overall design of coagulation tests created to assess procoagulant function but not anticoagulant function, have led to a situation where conventional coagulation tests do not reflect the actual coagulation status of patients in ESLD.21,22 Viscoelastic testing provides reliable information regarding all components of the coagulation system and should be used for assessment of coagulation in patients with ESLD.23,24
Fibrinolysis, or tertiary hemostasis, is a highly regulated mechanism by which fibrin is lysed by plasmin, thereby controlling excess fibrin formation. Plasmin's proenzyme plasminogen is activated under normal circumstances by tissue plasminogen activator (t-PA), urokinase plasminogen activator, and activated factor XII. These activators are balanced by inhibitors, such as PAI-1, plasmin inhibitor, and thrombin activatable fibrinolysis inhibitor.3 Fibrinolysis becomes hyperactive in up to 46% of liver cirrhosis patients.14 Plasminogen and antiplasmin levels are reduced in ESLD. Factor XIII and thrombin activatable fibrinolysis inhibitor concentrations are also lower in cirrhotic patients.15 Simultaneously, there are increased levels of t-PA (due to decreased metabolism in the liver) and PAI-1 (due to increased endothelial production).25 These changes in profibrinolytic and antifibrinolytic drivers most likely restore the balance of fibrinolysis in patients with liver disease to some extent,3 albeit with reduced reserve and a tendency to be tipped to one side or the other due to minor homeostatic perturbations. Reliable laboratory tests for global assessment of fibrinolysis are lacking.26
Clot stability also contributes to hypercoagulability in patients with ESLD. Recent work describes abnormal fibrin clot structure and function as a risk factor for thrombosis, including arterial and venous thromboembolic events.27 Hugenholtz et al28 demonstrated that the kinetics of clot structure were decreased, leading to slower clot formation with an associated decreased clot permeability in cirrhotic patients. These changes are probably due to modification of the fibrinogen molecule in an oxidative environment. Decreased clot permeability impairs thrombolytic activity, predisposing to thrombotic events.
The role of thrombophilic genetic factors is well established in venous thromboembolism (VTE), and a thrombophilic genotype may be present in upwards of 69.5% of cirrhotic patients who develop portal venous thrombosis (PVT).29 Coagulation factor V (FV) is a cofactor of the prothrombinase complex that activates thrombin leading to the formation of a blood clot. Factor V Leiden (FVL) is an autosomal dominant genetic mutation of FV where activated protein C is unable to inactivate the clotting factor.30 The prevalence of this condition is between 2% and 15% in the general population.31 Excessive clotting due to this disorder is mainly venous and it is the most common contributor to deep venous thrombosis (DVT).32 Homozygotes are at an increased risk versus heterozygotes: 50- to 100-fold versus fivefold to 10-fold increased risk of VTE, respectively.33 Patients with hepatitis C infection have an odds ratio (OR) of 4.0 of developing cirrhosis in the presence of FVL-related activated protein C resistance.34 Factor V Leiden increases the risk for arterial thrombosis. It has been demonstrated that recipient FVL is a risk factor for developing hepatic artery thrombosis (HAT) after LT. A study by Fan et al demonstrated a baseline risk for HAT after LT of 3.8% while 30% of patients with FVL developed this complication postoperatively.35
Prothrombin G20210A mutation (PTHR A20210) leads to elevated plasma prothrombin levels and is a moderate risk factor for venous thrombosis (OR, 2.8).36 Some data suggest a higher prevalence of PTHR A20210 in cirrhotic patients presenting with PVT, but a meta-analysis by Qi et al30 did not confirm this finding. High plasma homocysteine level is a known risk factor for venous thrombosis, and mutation in the methylenetetrahydrofolate reductase gene (MTHFR C677 → T) was found in 43.5% of cirrhotic patients with PVT, compared to 5% in those without PVT.29 In a larger study performed by the same group, the MTHFR mutation was not associated with PVT.37 Amitrano et al29 reported 2 cases of combined genetic defects leading to venous intestinal infarction in patients with liver cirrhosis. One patient had FVL + MTHFR C677 → T mutations and the other one had PTHR A20210 + MTHFR C677 → T mutations.
Myeloproliferative neoplasms (MPN) are common causes of PVT of noncirrhotic origin. These conditions, although rare, should be considered in cirrhosis patients with PVT.30 JAK2V617F mutations are present in 7% of patients with PVT and liver cirrhosis.38
Thrombophilic genetic mutations are more likely to predispose patients with ESLD to thrombotic events compared with the general population due to their hypercoagulable profile.
Antiphospholipid antibodies are commonly found in patients with primary biliary cirrhosis.39 Antiphospholipid antibodies are also frequently found in patients with Hepatitis C, where their presence may be due to immunologic effects of the hepatitis C virus (HCV), or due to prolonged tissue damage.40 Qi et al41 demonstrated in a meta-analysis an association between PVT in liver cirrhosis and anticardiolipin antibodies.
In conclusion, despite the decreased concentrations of both procoagulation and anticoagulation factors produced by the liver, hepatic failure does not necessarily lead to a balanced coagulation profile. In ESLD several pathophysiological mechanisms, especially profound ED, may be responsible for a hypercoagulable state with the potential for severe thrombotic complications.
ESLD AND VTE
Traditionally, patients with liver cirrhosis have been regarded as being at reduced risk for VTE.42 The evidence describing VTE risk has been conflicting. The majority of early evaluations were relatively small single-center studies that did not reflect a tendency for thrombosis associated with ESLD.43,44 Analyses of much larger populations from national and international databases were necessary to determine this association. One of the first significant evaluations was published in 2008 as a case-control study at a tertiary hospital, comparing 963 subjects with cirrhosis to 12 405 controls. Patients with cirrhosis did not have a lower risk of DVT or pulmonary embolism (PE) in comparison to controls.45 The risk for VTE in advanced liver disease was lower than in patients with congestive heart failure or cancer. In a retrospective analysis of the Danish National Registry, Søgaard et al demonstrated that chronic liver disease (CLD), both in the presence or absence of cirrhosis, was associated with a higher relative risk of unprovoked VTE compared to controls with ORs of 2.06 and 2.1 respectively.46 VTE in cirrhotic patients was associated with increased short term mortality. In a subsequent study, Søgaard's group found that adjusted 30-day mortality rate ratios were 2.17 and 1.83 for DVT and PE, respectively.47 In a similar nationwide analysis of hospitalized patients in the United States, Wu and Nguyen48 demonstrated that the OR for VTE was 1.23 in compensated cirrhosis, and 1.39 in decompensated cirrhosis, but only in those younger than 45 years of age. This study, using data from the National Inpatient Sample, also showed an increased mortality associated with VTE in cirrhotic patients (ORs, 2.16 and 1.6 in compensated cirrhosis and decompensated cirrhosis, respectively).
After these publications, the relationship between CLD and thromboembolism was widely recognized. The incidence of VTE in hospitalized patients with CLD varies between 0.5% and 8.2%.45,48-50 “Auto-anticoagulation” in the setting of an elevated INR in this population does not exist.4 Dabbagh et al50 described 190 subjects who developed VTE over a 7-year period. Notably, 51 of these subjects' INR was equal to or greater than 2.2.
ESLD AND PERIOPERATIVE THROMBOSIS
Preoperative
End-stage liver disease is associated with thrombosis at each stage of LT: preoperatively, intraoperatively, and postoperatively. Portal vein thrombosis (PVT) occurs most frequently preoperatively, with an incidence between 0.5% and 16%.4,48,51 Englesbe et al52 report a PVT incidence of 2% among patients on the LT waiting list. PVT among LT recipients was higher (4%) and was associated with a 30-day death hazard ratio (HR) of 1.5, and a 1-year mortality HR of 1.52.52 Singhal et al20 reported a PVT incidence of 12.8% in 47 consecutive patients with ESLD. An association between PVT and increased morbidity and mortality was demonstrated in several publications.51,53-55
Intraoperative
Intraoperative thrombosis is a serious complication associated with a very high mortality rate. The incidence of thromboembolic events in the form of intracardiac thrombus (ICT) or PE during LT ranges from 1% to 6%.56,57 ICT/PE has been described during all stages of LT, but most frequently around reperfusion.58 Predisposing factors are preoperative VTE, a transjugular intrahepatic portosystemic shunt (TIPS), veno-venous bypass during LT, presence of pulmonary artery and dialysis catheters, and exposure to antifibrinolytic agents. Intraoperative mortality after PE is 30% and in-hospital mortality is 45%.58 The relatively low incidence of intraoperative clotting events probably reflects the historically low utilization rate of intraoperative transesophageal echocardiography (TEE). However, TEE is routinely used, the incidence of thrombosis seems to be much higher, but not necessarily associated with hemodynamic instability. Schillcutt et al59 report abnormal TEE findings in 88% of patients in a retrospective analysis. 44% of patients demonstrated evidence of microemboli, 32% demonstrated ICT and 5% PE. The presence of ICT or biventricular dysfunction was predictive of major cardiac adverse events as well as increased mortality.
Postoperative
Post-LT thrombotic events manifest as VTE, PVT, and HAT. The incidence of posttransplant VTE is 5-10%, with a 4.6% incidence 1 year after LT.60 Post-LT PVT is a relatively infrequent complication with an incidence about 2% and can be associated with preoperative PVT.61 Acute postoperative PVT a high morbidity and mortality, often necessitating urgent redo LT. Postoperative HAT is classified as early (within the first 90 days after LT depending on the particular author) or late. Late HAT does not usually lead to graft failure, but early postoperative HAT frequently requires urgent retransplantation.61 Pretransplant PVT is associated with posttransplant thrombosis.53,61,62 Hepatic artery thrombosis is present in more than 6% of patients after LT, is more common in pediatric LT, and is frequently associated with graft failure and increased mortality.63 Although technical factors play a role in the development of HAT, nonsurgical factors such as low recipient weight, sclerosing cholangitis, cytomegalovirus recipient/donor mismatch, retransplantation, and prolonged cold ischemic time are also associated with increased rates of early HAT.64 In a prospective study by Eldeen et al,65 828 patients underwent LT and 79 patients (9.5%) developed HAT. Twenty-three of these patients developed early HAT—defined by the authors as occurrence within 21 days of transplant. A maximum amplitude value of 65 mm or greater on preoperative thromboelastography carried a hazard ratio of 5.28 of developing early HAT.
ESLD POPULATIONS SUSCEPTIBLE TO THROMBOTIC EVENTS
The association between ESLD and hypercoagulability is well recognized. There are, however, special populations that are at even greater risk in addition to the risk from ESLD.
Nonalcoholic Steatohepatitis
PE/DVT and PVT appear to occur more frequently in patients with liver cirrhosis due to nonalcoholic steatohepatitis (NASH), independently of other factors predisposed to thrombosis such as diabetes and obesity.66-68 Chronic inflammation in this condition leads to oxidative injury and ED with subsequent alteration in hemostatic balance. Major contributors to this procoagulant state are increased concentrations of several factors including FIX, FXI, and FXIII as well as reduced protein C levels. FVIII, vWF and PAI-1 are increased to a greater degree than in other patients with ESLD.69,70 Potze et al71 challenge this view, suggesting that the increased risk for thrombotic events in NASH-related cirrhosis is probably not due to altered hemostasis but rather due to hypofibrinolysis and altered fibrin clot structure associated with obesity. Despite this, multiple other studies have shown that the risk of VTE in NASH persists even when adjusting for confounding metabolic factors including obesity.68,72
Autoimmune Conditions
Bezinover et al62 demonstrated that causes of ESLD related to autoimmune conditions predispose patients to perioperative thrombosis. In their retrospective database analysis, autoimmune hepatitis was associated with both preoperative PVT and postoperative thrombotic complications. Primary biliary cirrhosis and primary sclerosing cholangitis were associated mostly with postoperative thrombosis. The cause of this association is likely chronic inflammation, along with increased release of cytokines. Fibrinogen and tissue factor concentrations are higher in these patients than in those with ESLD due to other causes.73,74
Chronic Hepatitis C
Chronic HCV infection may be a predisposing factor for thrombosis. It has been demonstrated that HCV results in a persistent inflammatory state leading to liver fibrosis.75 Chronic HCV infection also leads to the production of various auto-antibodies such anticardiolipins and is one of the causes of antiphospholipid syndrome.76 Thrombin is generated at significantly higher levels in HCV patients.77 The predisposition to thromboses of patients with HCV remains, however, controversial and needs further evaluation.
Race and Ethnic Groups
In the general population, the prevalence of perioperative thrombotic events is higher in patients of black or white race, whereas it is lower in Asian patients.78-80 In general, African Americans (AA) are predisposed to VTE events due to higher levels of factor VIII and vWF and lower protein C levels.80 However, in patients with ESLD, a completely different distribution of PVT was demonstrated. The AA population had the lowest prevalence of PVT in comparison to other ethnic groups, and the Hispanic population had the highest.68,81 The reason for the low prevalence of thrombosis in the AA population with ESLD is not completely understood, but a genetic predisposition to decreased portal pressure in the AA population is currently under discussion.81
Association between Pre and Postoperative Thrombosis
Papers based on a nationwide U.S. transplant database evaluation clearly demonstrated an association between preoperative PVT and postoperative HAT.53,61,62 The cause of this phenomenon can be related to the fact that hypercoagulability seen preoperatively does not resolve immediately after transplantation.82 Also, ongoing post-LT ED69 in combination with prolonged cold ischemic time and technically demanding surgical anastomoses,53 makes patients with PVT predisposed to postoperative thrombotic complications.
Patients with conditions described above are potentially prone to thrombotic events. Routine antithrombotic prophylaxis in these susceptible populations is a frequent consideration.
ANTICOAGULATION IN ESLD
Heparin
The use of unfractionated heparin (UFH) and low-molecular weight heparin (LMWH) in ESLD is well described.83-85 Liver cirrhosis causes decreased antifactor Xa (anti-Xa) activity, and concurrent increased INR and activated partial thromboplastin time.86 This makes monitoring of heparin therapy very challenging.87 Uchikawa et al88 demonstrated that activated clotting time (ACT) measurement is a reliable method for monitoring of LMWH anticoagulant effects during living donor LT. The ACT level should be kept within normal range to prevent hemorrhagic complications. This may be impractical when baseline ACT is already elevated in severe liver disease. ESLD is associated with decreased ATIII levels, possibly rendering UFH and LMWH less effective, whereas lower anti-Xa levels might still be adequate to produce clot.89 Reduced UFH and LMWH effectivity may explain why Moorehead et al found that VTE prophylaxis using UFH or LMWH was not associated with a lower risk of VTE or an increased risk of bleeding.90 There are several problems related to the use of these agents. UFH and LMWH are administered parenterally reducing patient compliance.91 Concern for heparin-induced thrombocytopenia (HIT) and skin reactions may limit long-term use of UFH and LMWH. The incidence of HIT in hospitalized patients receiving thromboprophylaxis is 0.5%.92 Heparin-induced thrombocytopenia has been described in a cirrhosis patient on therapeutic LMWH,93 but the risk of HIT is unknown when prophylactic doses of UFH or LMWH are used.84 LMWH may accumulate in renal failure, complicating its use in patients with hepatorenal syndrome. UFH may be a better option in patients with renal failure.86
Vitamin K Antagonists
Vitamin K antagonists (VKA) have a narrow therapeutic margin and require regular monitoring as well as careful attention to diet. In liver disease, the therapeutic margin may be reduced due to alterations in pharmacokinetics related to absorption, volumes of distribution, and protein binding. Vitamin K antagonists are difficult to monitor in liver cirrhosis because of baseline changes in INR, as well as high between-laboratory variability in the INR of cirrhotic patients,87 putting them at risk for overdosing as well as underdosing.94 Selection of a target INR range for VKA therapy is frequently empirical in this population.91 Altering INR increases a patient's Model for End Stage Liver Disease score, with subsequent organ allocation implications. Alternative reliable monitoring tests for VKA use in cirrhosis have not been established. Despite all the above deficiencies, Kuo et al demonstrated, in a retrospective case-control study using the Taiwanese National Health Insurance Research Database, that warfarin use in cirrhosis subjects with atrial fibrillation was effective for prevention of ischemic stroke compared to no intervention.95 A total of 9056 subjects with cirrhosis were included in this study, of which 754 were treated with warfarin. These subjects had fewer complications such as encephalopathy and variceal bleeding. This may be due to selection bias, as subjects on warfarin had fewer comorbidities, as reflected by their CHA2DS2VASc scores, compared to those receiving no intervention.
Direct Oral Anticoagulants
Direct oral anticoagulants (DOAC) are attractive options for use in ESLD. Experience with DOAC in cirrhotic patients is limited because large clinical DOAC trials excluded patients with ESLD. These agents inhibit single coagulation proteases and function independently of ATIII. Rivaroxaban, apixaban and edoxaban are direct factor Xa inhibitors, cleared by the liver and kidneys. Rivaroxaban is not recommended for use in patients with Child-Turcotte-Pugh (CTP) class B and C cirrhosis due to reduced anticoagulant effect in vitro, while anticoagulation potency in CTP A and B patients are similar to controls.96 DOACs do not induce HIT and do not require frequent laboratory monitoring, although commercially available drug levels are expected in the near future.87 Clinically, major bleeding rates appear to be similar to rates in patients receiving traditional anticoagulation, but more research is required.97 Dabigatran, a direct factor IIa inhibitor, is eliminated mainly by the kidneys. Moderate liver impairment does not affect its pharmacokinetics,98 although accumulation in kidney failure requires dosage adjustment.99 Potze et al96 demonstrated in an in vitro study that the anticoagulant effect may be higher in patients with ESLD but this needs to be confirmed by clinical studies. The anticoagulant effect of DOACs may be reversed by prothrombin complex concentrates, or dialysis in the case of dabigatran.100 Idarucizumab is the only agent approved by the US Food and Drug Administration to reverse the effects of dabigatran.101 Intagliata et al102 describe the successful use of idarucizumab to reverse the anticoagulant effects of dabigatran during LT, which was completed safely with neither bleeding nor thrombosis. Andexanet alfa, a reversal agent for rivaroxaban and apixaban, was also recently approved by the Food and Drug Administration, however, use in cirrhosis patients has not been described. The risk of bleeding in cirrhosis patients using DOACs seems to be similar to when traditional anticoagulants are used.97 Apixaban and edoxaban appear to be safe in CTP Class A and B patients.97,103,104 Anticoagulation with apixaban is less effective in CTP C patients,105 and little is known about edoxaban and CTP C patients.91 The first approved factor IIa inhibitor, ximelatragan, was withdrawn due to hepatotoxicity.106 Currently available DOACs seem to pose little risk of drug-induced liver injury.107 In the meantime, we eagerly await the results from the CIRROXABAN trial. (https://clinicaltrials.gov/ct2/show/NCT02643212)
Aspirin
Nonsteroidal antiinflammatory drugs are generally avoided in both compensated and decompensated liver cirrhosis due to the risk of acute renal failure.108 There is also a correlation between nonsteroidal antiinflammatory drugs use and bleeding from esophageal varices.109 Patients with ascites may develop acute renal failure, hyponatremia, and diuretic resistance in response to aspirin treatment.110 Aspirin use appears to be safe in cirrhosis patients without significant varices after coronary stenting.111 Successful use of aspirin for prevention of posttransplant HAT has been described,112 however other studies have not confirmed these findings.113,114
PERIOPERATIVE ANTITHROMBOTIC MANAGEMENT
Portal Vein Thrombosis (PVT)
PVT Prophylaxis
To date, the topic of PVT prophylaxis remains controversial and relatively unexplored. Consequently, consensus guidelines are lacking.115 A study by Villa et al demonstrating safe prevention of PVT using prophylactic LMWH dosing is promising as the authors demonstrated not only PVT prevention, but also reduced bacterial translocation, incidence of hepatic decompensation, and improved survival.116 Others are of the opinion that the data are insufficient to justify widespread primary pharmacological PVT prophylaxis.87 PVT prophylaxis may be indicated in cirrhotic patients awaiting LT or after hepatic resection.117
Recommendation
PVT prophylaxis cannot be recommended for or against based on the currently available literature and may be considered on an individual case by case basis at the discretion of the treating physician (level of evidence III, recommendation grade C—see Table 1).
TABLE 1.
Classification schemes for levels of evidence and recommendations
Treatment of Established PVT
Similar to PVT prevention, the treatment of PVT offers controversy and the majority of consensus opinion focuses on patients who are candidates for LT as most studies guiding clinical decision making have been retrospective with significant limitations. Despite this, bleeding rates are acceptable and, in general, are similar to what is expected in an acutely ill medical patient population.89,93,97,118-127 Rates of minor bleeding are from 1-13% and major bleeding, including variceal hemorrhage, intracranial hemorrhage, and retroperitoneal hemorrhage, are 5-20%. It is important to note that there is significant heterogeneity in the definition of bleeding events which limits accuracy across the literature. A recent meta-analysis of observational anticoagulation treatment trials for PVT utilizing LMWH or VKAs128 demonstrated a 3.3% pooled bleeding rate (both major and minor events) (95% CI 1.1-6.7%). Furthermore, anticoagulation itself has previously been shown to not be predictive of upper gastrointestinal bleeding events, both variceal and nonvariceal.129
In general, treatment with anticoagulation should be considered in all patients with CTP Class A or B cirrhosis. VKAs, LMWH or DOACs may all be considered in coordination with hematology consultation. VKAs are limited by the fact that patients with cirrhosis often have elevated PT-INR values at baseline. Consequently, smaller doses of VKAs are required to obtain the consensus therapeutic window, and frequent careful monitoring, which inconveniences the patient and places burden on the healthcare system, are standard. LMWH is similarly limited in practicality as daily injections require patient education and availability, and proper storage of medical supplies. Furthermore, using anti-Xa levels to guide dosing of LMWH is problematic in patients with cirrhosis as patients often fail to achieve the desirable therapeutic levels of anti-Xa with either prophylactic or therapeutic dosing.89 LMWH also does not have a reversal agent. DOACs, while widely used in cardiovascular and hematologic disease with favorable safety and efficacy profiles, are largely experimental in patients with cirrhosis. Although bleeding rates are similar when comparing VKAs or LMWH use to DOACs for PVT treatment,97 clinicians have been hesitant to use DOACs for this purpose. Prothrombin complex concentrates may be considered as a surrogate for a direct reversal agent to normalize PT while the newer reversal agents are awaited.130,131
Historically, TIPS has been considered to be contraindicated in patients with cirrhosis and PVT. However, more recent reports have surfaced and TIPS is in fact a safe and effective treatment for chronic PVT complicated by significant portal hypertension or symptomatic complete occlusion of the main portal vein.132-134 Rates of recanalization are similar to or better than that published for anticoagulation alone, ranging from 60-92%, depending on procedural technique (eg, transsplenic vs transjugular) and TIPS can be considered an equivalent alternative to chronic anticoagulation.135-137 TIPS is often undertaken with concomitant anticoagulation and is often institution dependent, however recent reports suggest that TIPS alone may be enough for recanalization without chronic anticoagulation.136-138 Whether or not anticoagulation or TIPS is utilized, the ultimate goal is for either to serve as a bridge to transplantation to keep the portal vein patent, allowing the surgical graft to be constructed.
Recommendation
The use of anticoagulation for treatment of established PVT can be recommended on an individual basis with a multidisciplinary approach in collaboration with a hematologist. There are insufficient data on the choice of one anticoagulant over another. Caution should be used in advanced liver disease with CPT Class B or C. (Level III, Grade C). TIPS is an effective treatment for chronic PVT in patients with cirrhosis and portal hypertension (Level II, Grade B).
Venous Thromboembolism (VTE)
Deep vein Thrombosis (DVT) and PE Prophylaxis
VTE complications are associated with increased hospital length of stay and cost, leading to increased healthcare burden, in addition to inferior outcomes.139 Primary prevention of VTE in the general population is achieved with LMWH, fondaparinux, UFH, or anti Xa and IIa inhibitors.87 In a study published in 2006, only 21% of hospitalized patients with cirrhosis received thromboprophylaxis.4 A third of these patients received pharmacological prophylaxis, whereas the remainder received mechanical thromboprophylaxis. A more recent report found that 44% of patients with CLD received pharmacological prophylaxis,140 suggesting an increasing awareness about the risk for VTE in these patients.
Prospective literature regarding VTE anticoagulation in patients with ESLD is scarce, mostly based on data from patients with PVT,141 and appears to be safe in this population.89,90 Lisman et al87 are of the opinion that patients with ESLD should receive LMWH prophylaxis in all situations where patients without liver disease would receive thromboprophylaxis, such as hospitalization, postsurgery and immobilization. Vivarelli et al demonstrated that the presence of esophageal varices was the only independent risk factor for bleeding complications in patients with cirrhosis who received VTE prophylaxis after liver resection for hepatocellular carcinoma.142 Mechanical thromboprophylaxis (in the form of pneumatic compression devices or compression stockings) should be offered to patients with contraindications to anticoagulation. Hospitalized cirrhosis patients should have individualized plans for pharmacological VTE prophylaxis.90
Recommendations
Hospitalized and immobilized patients with cirrhosis without contraindication should receive VTE prophylaxis with either LMWH or UFH. (Level III, Grade C).
Treatment of DVT and PE
As the majority of anticoagulation studies in cirrhosis have evaluated PVT, clinical recommendations for the treatment of PE and DVT are often extrapolated from this similar area of research. One retrospective study involving therapeutic anticoagulation in 17 cirrhosis patients with VTE not associated with PVT (11 patients with LMWH and 6 patients with LMWH + acenocoumarol) 14 patients (83%) developed hemorrhagic complications, of which 6 (35%) required blood transfusions.143 Two recent prospective studies report a low bleeding risk in patients with PVT on therapeutic anticoagulation.93,125 Lisman et al87 suggest initiating treatment with LMWH, transitioning to VKA or maintaining long-term LMWH therapy. The optimal dosing regime is unknown. Conservative dosing is recommended, especially in patients with severe thrombocytopenia or renal failure. A recent experience from Intagliata et al97 examined patients with cirrhosis treated with DOAC versus traditional (LMWH or VKA) and found no difference in bleeding rates when comparing the 2 groups. Of the 39 patients who were, 16 had VTE.97 There was no difference between cohorts in bleeding events (4 [20%] of 20 in DOAC group versus 3 [16%] of 19 in traditional anticoagulant group).
Recommendation
Patients with cirrhosis and VTE should be treated with anticoagulation similar to other medical patients. There is no agent of choice and LMWH, UFH, VKAs, and DOACs can be considered on an individual basis (level III, grade C).
Acute Intraoperative Thromboses
There are many reports describing ICT or PE during LT.56,57,144-146 These cases are usually associated with dramatic changes in hemodynamics and frequently have bad outcomes. Due to more common use of intraoperative TEE, ICT seems to be a relatively frequent finding in patients undergoing LT. Intracardiac thrombus as detected by TEE is not necessarily associated with hemodynamic instability, nor does it always require treatment.59,146 There are only a few reports of successful management of symptomatic ICT or PE during LT. Techniques used to treat ICT and PE include the use of recombinant t-PA (rTPA), thrombectomy, extracorporeal circulatory support, and intravenous heparin with various degrees of success.145,147,148 In early publications, acute PE with circulatory compromise was treated with 40 to 100 mg of intravenous rTPA administered over 2 hours.149,150 This treatment is associated with hemorrhage, which may be severe during LT. Recombinant t-PA doses of 0.5 to 4 mg administered through a central vein during LT appear to be safe and effective, albeit with increased transfusion requirements.145 Protin et al146 propose an algorithm based on the size and progression of an ICT visualized by TEE. Management options include watchful waiting, heparin infusion, and rTPA administration. More prospective research is needed to guide management of this potentially devastating condition.
Recommendation
Routine use of TEE may help early recognition and treatment of intraoperative thrombosis before significant hemodynamic instability occurs. Administration of heparin should be reserved for ICT/PE without hemodynamic instability. In an unstable patient, doses of rTPA 0.5 to 4 mg and higher (depending of degree of instability) can be used (level IV, grade D).
HAT
Hepatic artery thrombosis is diagnosed by hepatic artery palpation or Doppler ultrasonography. Restoration of graft function requires immediate surgical arterial thrombectomy and reconstruction.151 In liver recipients at risk for HAT, thromboprophylactic strategies with heparin or aspirin may be beneficial.152 The use of UFH and LMWH to reduce the incidence of HAT in high risk populations such as pediatric and living donor LT is described.153,154 Reports of use of heparin during deceased donor LT are limited. Aspirin's role in preventing early HAT seems unclear. Wolf et al report that low-dose aspirin therapy (up to 81 mg per day) does not reduce early HAT incidence,113 whereas higher dose aspirin (325 mg per day) reduced the incidence of early HAT from 3.9% to 1.8%.155 Both studies report a low incidence of significant bleeding. The risk of late HAT seems to be substantially decreased in patients receiving aspirin (3.6% vs 0.6%).112 Prospective randomized trials are lacking. In addition, pretransplant PVT is associated with posttransplant HAT within the first 90 days after liver transplant.53,156 Whether or not use of an anticoagulant in a patient with pretransplant PVT can prevent posttransplant HAT remains unknown.
Recommendation
Prophylactic administration of UFH and LMWH in the immediate perioperative period, to reduce the incidence of HAT in liver transplant recipients at high-risk for HAT, should be considered on an individual basis. Recommendations for or against chronic aspirin use to prevent HAT cannot be made at this time. (level of evidence III, grade C).
CONCLUSIONS
The historical concept that patients with ESLD are auto-anticoagulated is no longer valid. ESLD patients have reduced hemostatic reserve, which can manifest as hypercoagulability as much as, if not more often, than bleeding. Hospitalized cirrhotic patients are at an increased risk of thrombotic events and should be considered for thromboprophylaxis—mechanical and/or pharmacological. There is a paucity of data regarding thromboprophylaxis and treatment of VTE in cirrhotic patients. Clinical practice is based on data from the general population as well as from ESLD patients with PVT. Anticoagulation appears to be a safe and effective treatment for PVT on an individual basis, but cannot be universally recommended. Intracardiac thrombosis during LT and HAT are thrombotic complications unique to LT that require urgent intervention. In this new era of heightened awareness of thrombotic events in ESLD patients, prospective randomized trials are urgently needed to best guide clinical practice.
Footnotes
Published online 26 October, 2018.
The authors declare no funding or conflicts of interest.
T.A.V. participated in literature review, article preparation, and review of the article. J.G.S. participated in literature review, article preparation, and review of the article. F.H.S. participated in literature review, article preparation, and review of article. D.B. participated in literature review, article preparation, review of the article.
REFERENCES
- 1.Tripodi A, Salerno F, Chantarangkul V, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553–558. [DOI] [PubMed] [Google Scholar]
- 2.de Boer MT, Molenaar IQ, Hendriks HG, et al. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265–275. [DOI] [PubMed] [Google Scholar]
- 3.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. [DOI] [PubMed] [Google Scholar]
- 4.Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101:1524–1528; quiz 1680. [DOI] [PubMed] [Google Scholar]
- 5.Shekelle PG, Woolf SH, Eccles M, et al. Clinical guidelines: developing guidelines. BMJ. 1999;318:593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vairappan B. Endothelial dysfunction in cirrhosis: role of inflammation and oxidative stress. World J Hepatol. 2015;7:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violi F, Ferro D, Basili S, et al. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost. 1997;77:44–47. [PubMed] [Google Scholar]
- 8.Oksüzoğlu G, Simsek H, Haznedaroğlu IC, et al. Tissue factor pathway inhibitor concentrations in cirrhotic patients with and without portal vein thrombosis. Am J Gastroenterol. 1997;92:303–306. [PubMed] [Google Scholar]
- 9.Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–178. [DOI] [PubMed] [Google Scholar]
- 10.Riordan SM, Skinner N, Nagree A, et al. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154–1164. [DOI] [PubMed] [Google Scholar]
- 11.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. [DOI] [PubMed] [Google Scholar]
- 12.Mookerjee RP, Vairappan B, Jalan R. The puzzle of endothelial nitric oxide synthase dysfunction in portal hypertension: the missing piece? Hepatology. 2007;46:943–946. [DOI] [PubMed] [Google Scholar]
- 13.Hollestelle MJ, Geertzen HG, Straatsburg IH, et al. Factor VIII expression in liver disease. Thromb Haemost. 2004;91:267–275. [DOI] [PubMed] [Google Scholar]
- 14.Leebeek FW, Kluft C, Knot EA, et al. A shift in balance between profibrinolytic and antifibrinolytic factors causes enhanced fibrinolysis in cirrhosis. Gastroenterology. 1991;101:1382–1390. [DOI] [PubMed] [Google Scholar]
- 15.Saner FH, Gieseler RK, Akiz H, et al. Delicate balance of bleeding and thrombosis in end-stage liver disease and liver transplantation. Digestion. 2013;88:135–144. [DOI] [PubMed] [Google Scholar]
- 16.Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53–61. [DOI] [PubMed] [Google Scholar]
- 17.Laffi G, Cominelli F, Ruggiero M, et al. Altered platelet function in cirrhosis of the liver: impairment of inositol lipid and arachidonic acid metabolism in response to agonists. Hepatology. 1988;8:1620–1626. [DOI] [PubMed] [Google Scholar]
- 18.Mannucci PM, Canciani MT, Forza I, et al. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98:2730–2735. [DOI] [PubMed] [Google Scholar]
- 19.Raparelli V, Basili S, Carnevale R, et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology. 2017;65:571–581. [DOI] [PubMed] [Google Scholar]
- 20.Singhal A, Karachristos A, Bromberg M, et al. Hypercoagulability in end-stage liver disease: prevalence and its correlation with severity of liver disease and portal vein thrombosis. Clin Appl Thromb Hemost. 2012;18:594–598. [DOI] [PubMed] [Google Scholar]
- 21.Lisman T, Bakhtiari K, Pereboom IT, et al. Normal to increased thrombin generation in patients undergoing liver transplantation despite prolonged conventional coagulation tests. J Hepatol. 2010;52:355–361. [DOI] [PubMed] [Google Scholar]
- 22.Ewe K. Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci. 1981;26:388–393. [DOI] [PubMed] [Google Scholar]
- 23.Mallett SV, Chowdary P, Burroughs AK. Clinical utility of viscoelastic tests of coagulation in patients with liver disease. Liver Int. 2013;33:961–974. [DOI] [PubMed] [Google Scholar]
- 24.Bezinover D, Dirkmann D, Findlay J, et al. Perioperative coagulation management in liver transplant recipients. Transplantation. 2018;102:578–592. [DOI] [PubMed] [Google Scholar]
- 25.Senzolo M, Burra P, Cholongitas E, et al. New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol. 2006;12:7725–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripodi A, Anstee QM, Sogaard KK, et al. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9:1713–1723. [DOI] [PubMed] [Google Scholar]
- 27.Undas A. Fibrin clot properties and their modulation in thrombotic disorders. Thromb Haemost. 2014;112:32–42. [DOI] [PubMed] [Google Scholar]
- 28.Hugenholtz GC, Macrae F, Adelmeijer J, et al. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054–1066. [DOI] [PubMed] [Google Scholar]
- 29.Amitrano L, Brancaccio V, Guardascione MA, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology. 2000;31:345–348. [DOI] [PubMed] [Google Scholar]
- 30.Qi X, Li H, Liu X, et al. Novel insights into the development of portal vein thrombosis in cirrhosis patients. Expert Rev Gastroenterol Hepatol. 2015;9:1421–1432. [DOI] [PubMed] [Google Scholar]
- 31.Dahlbäck B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 2008;112:19–27. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Pujol S, Aras O, Escolar G. Factor v leiden and inflammation. Thrombosis. 2012;2012:594986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Stefano V, Chiusolo P, Paciaroni K, et al. Epidemiology of factor V Leiden: clinical implications. Semin Thromb Hemost. 1998;24:367–379. [DOI] [PubMed] [Google Scholar]
- 34.Poujol-Robert A, Boëlle PY, Poupon R, et al. Factor V Leiden as a risk factor for cirrhosis in chronic hepatitis C. Hepatology. 2004;39:1174–1175. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Nishida S, Selvaggi G, et al. Factor V Leiden mutation is a risk factor for hepatic artery thrombosis in liver transplantation. Transplant Proc. 2013;45:1990–1993. [DOI] [PubMed] [Google Scholar]
- 36.Walker AP. Portal vein thrombosis: what is the role of genetics? Eur J Gastroenterol Hepatol. 2005;17:705–707. [DOI] [PubMed] [Google Scholar]
- 37.Amitrano L, Guardascione MA, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736–741. [DOI] [PubMed] [Google Scholar]
- 38.Qi X, Yang Z, Bai M, et al. Meta-analysis: the significance of screening for JAK2V617F mutation in Budd-Chiari syndrome and portal venous system thrombosis. Aliment Pharmacol Ther. 2011;33:1087–1103. [DOI] [PubMed] [Google Scholar]
- 39.Mankaï A, Manoubi W, Ghozzi M, et al. High frequency of antiphospholipid antibodies in primary biliary cirrhosis. J Clin Lab Anal. 2015;29:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangia A, Margaglione M, Cascavilla I, et al. Anticardiolipin antibodies in patients with liver disease. Am J Gastroenterol. 1999;94:2983–2987. [DOI] [PubMed] [Google Scholar]
- 41.Qi X, De Stefano V, Su C, et al. Associations of antiphospholipid antibodies with splanchnic vein thrombosis: a systematic review with meta-analysis. Medicine (Baltimore). 2015;94:e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. [DOI] [PubMed] [Google Scholar]
- 43.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–815. [DOI] [PubMed] [Google Scholar]
- 44.Huerta C, Johansson S, Wallander MA, et al. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–943. [DOI] [PubMed] [Google Scholar]
- 45.Gulley D, Teal E, Suvannasankha A, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53:3012–3017. [DOI] [PubMed] [Google Scholar]
- 46.Søgaard KK, Horváth-Puhó E, Grønbaek H, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96–101. [DOI] [PubMed] [Google Scholar]
- 47.Søgaard KK, Horváth-Puhó E, Montomoli J, et al. Cirrhosis is associated with an increased 30-day mortality after venous thromboembolism. Clin Transl Gastroenterol. 2015;6:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8:800–805. [DOI] [PubMed] [Google Scholar]
- 49.Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064–1074. [DOI] [PubMed] [Google Scholar]
- 50.Dabbagh O, Oza A, Prakash S, et al. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137:1145–1149. [DOI] [PubMed] [Google Scholar]
- 51.Pareja E, Cortes M, Navarro R, et al. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 2010;42:2970–2972. [DOI] [PubMed] [Google Scholar]
- 52.Englesbe MJ, Schaubel DE, Cai S, et al. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010;16:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stine JG, Pelletier SJ, Schmitt TM, et al. Pre-transplant portal vein thrombosis is an independent risk factor for graft loss due to hepatic artery thrombosis in liver transplant recipients. HPB (Oxford). 2016;18:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chardot C, Herrera JM, Debray D, et al. Portal vein complications after liver transplantation for biliary atresia. Liver Transpl Surg. 1997;3:351–358. [DOI] [PubMed] [Google Scholar]
- 55.Stine JG, Shah PM, Cornella SL, et al. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: a meta-analysis. World J Hepatol. 2015;7:2774–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gologorsky E, De Wolf AM, Scott V, et al. Intracardiac thrombus formation and pulmonary thromboembolism immediately after graft reperfusion in 7 patients undergoing liver transplantation. Liver Transpl. 2001;7:783–789. [DOI] [PubMed] [Google Scholar]
- 57.Lerner AB, Sundar E, Mahmood F, et al. Four cases of cardiopulmonary thromboembolism during liver transplantation without the use of antifibrinolytic drugs. Anesth Analg. 2005;101:1608–1612. [DOI] [PubMed] [Google Scholar]
- 58.Sakai T, Matsusaki T, Dai F, et al. Pulmonary thromboembolism during adult liver transplantation: incidence, clinical presentation, outcome, risk factors, and diagnostic predictors. Br J Anaesth. 2012;108:469–477. [DOI] [PubMed] [Google Scholar]
- 59.Shillcutt SK, Ringenberg KJ, Chacon MM, et al. Liver transplantation: intraoperative transesophageal echocardiography findings and relationship to major postoperative adverse cardiac events. J Cardiothorac Vasc Anesth. 2016;30:107–114. [DOI] [PubMed] [Google Scholar]
- 60.Salami A, Qureshi W, Kuriakose P, et al. Frequency and predictors of venous thromboembolism in orthotopic liver transplant recipients: a single-center retrospective review. Transplant Proc. 2013;45:315–319. [DOI] [PubMed] [Google Scholar]
- 61.Duffy JP, Hong JC, Farmer DG, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896–903; discussion 903-5. [DOI] [PubMed] [Google Scholar]
- 62.Bezinover D, Iskandarani K, Chinchilli V, et al. Autoimmune conditions are associated with perioperative thrombotic complications in liver transplant recipients: a UNOS database analysis. BMC Anesthesiol. 2016;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iida T, Kaido T, Yagi S, et al. Hepatic arterial complications in adult living donor liver transplant recipients: a single-center experience of 673 cases. Clin Transplant. 2014;28:1025–1030. [DOI] [PubMed] [Google Scholar]
- 64.Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746–757. [DOI] [PubMed] [Google Scholar]
- 65.Zahr Eldeen F, Roll GR, Derosas C, et al. Preoperative thromboelastography as a sensitive tool predicting those at risk of developing early hepatic artery thrombosis after adult liver transplantation. Transplantation. 2016;100:2382–2390. [DOI] [PubMed] [Google Scholar]
- 66.Stine JG, Northup PG. Coagulopathy before and after liver transplantation: from the hepatic to the systemic circulatory systems. Clin Liver Dis. 2017;21:253–274. [DOI] [PubMed] [Google Scholar]
- 67.Stine JG, Niccum BA, Zimmet AN, et al. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin Transl Gastroenterol. 2018;9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stine JG, Shah NL, Argo CK, et al. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21:1016–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tripodi A, Fracanzani AL, Primignani M, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61:148–154. [DOI] [PubMed] [Google Scholar]
- 70.Kotronen A, Joutsi-Korhonen L, Sevastianova K, et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011;31:176–183. [DOI] [PubMed] [Google Scholar]
- 71.Potze W, Siddiqui MS, Boyett SL, et al. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;65:980–987. [DOI] [PubMed] [Google Scholar]
- 72.Ghabril M, Chalasani N. The Authors' Response. Transplantation. 2017;101:e282–e283. [DOI] [PubMed] [Google Scholar]
- 73.Zoller B, Li X, Sundquist J, et al. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2:171–183. [PMC free article] [PubMed] [Google Scholar]
- 74.Biagini MR, Tozzi A, Marcucci R, et al. Hyperhomocysteinemia and hypercoagulability in primary biliary cirrhosis. World J Gastroenterol. 2006;12:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zampino R, Marrone A, Restivo L, et al. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prieto J, Yuste JR, Beloqui O, et al. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology. 1996;23:199–204. [DOI] [PubMed] [Google Scholar]
- 77.Violi F, Ferro D, Basili S, et al. Increased rate of thrombin generation in hepatitis C virus cirrhotic patients. Relationship to venous thrombosis. J Investig Med. 1995;43:550–554. [PubMed] [Google Scholar]
- 78.Liao S, Woulfe T, Hyder S, et al. Incidence of venous thromboembolism in different ethnic groups: a regional direct comparison study. J Thromb Haemost. 2014;12:214–219. [DOI] [PubMed] [Google Scholar]
- 79.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S11–S17. [DOI] [PubMed] [Google Scholar]
- 80.Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9:1877–1882. [DOI] [PubMed] [Google Scholar]
- 81.Bezinover D, Reeder E, Aziz F, et al. African Americans have a lower prevalence of portal vein thrombosis at the time of liver transplantation. HPB (Oxford). 2017;19:620–628. [DOI] [PubMed] [Google Scholar]
- 82.Arshad F, Lisman T, Porte RJ. Hypercoagulability as a contributor to thrombotic complications in the liver transplant recipient. Liver Int. 2013;33:820–827. [DOI] [PubMed] [Google Scholar]
- 83.Smith CB, Hurdle AC, Kemp LO, et al. Evaluation of venous thromboembolism prophylaxis in patients with chronic liver disease. J Hosp Med. 2013;8:569–573. [DOI] [PubMed] [Google Scholar]
- 84.Intagliata NM, Henry ZH, Shah N, et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34:26–32. [DOI] [PubMed] [Google Scholar]
- 85.Shatzel J, Dulai PS, Harbin D, et al. Safety and efficacy of pharmacological thromboprophylaxis for hospitalized patients with cirrhosis: a single-center retrospective cohort study. J Thromb Haemost. 2015;13:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ha NB, Regal RE. Anticoagulation in patients with cirrhosis: caught between a rock-liver and a hard place. Ann Pharmacother. 2016;50:402–409. [DOI] [PubMed] [Google Scholar]
- 87.Lisman T, Kamphuisen PW, Northup PG, et al. Established and new-generation antithrombotic drugs in patients with cirrhosis—possibilities and caveats. J Hepatol. 2013;59:358–366. [DOI] [PubMed] [Google Scholar]
- 88.Uchikawa Y, Ikegami T, Masuda Y, et al. Administration of dalteparin based on the activated clotting time for prophylaxis of hepatic vessel thrombosis in living donor liver transplantation. Transplant Proc. 2009;41:3784–3790. [DOI] [PubMed] [Google Scholar]
- 89.Bechmann LP, Sichau M, Wichert M, et al. Low-molecular-weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31:75–82. [DOI] [PubMed] [Google Scholar]
- 90.Moorehead KJ, Jeffres MN, Mueller SW. A retrospective cohort analysis of pharmacologic VTE prophylaxis and padua prediction score in hospitalized patients with chronic liver disease. J Pharm Pract. 2017;30:58–63. [DOI] [PubMed] [Google Scholar]
- 91.Intagliata NM, Maitland H, Caldwell SH. Direct oral anticoagulants in cirrhosis. Curr Treat Options Gastroenterol. 2016;14:247–256. [DOI] [PubMed] [Google Scholar]
- 92.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–2715. [DOI] [PubMed] [Google Scholar]
- 93.Delgado MG, Seijo S, Yepes I, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–783. [DOI] [PubMed] [Google Scholar]
- 94.Hum J, Shatzel JJ, Jou JH, et al. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98:393–397. [DOI] [PubMed] [Google Scholar]
- 95.Kuo L, Chao TF, Liu CJ, et al. Liver cirrhosis in patients with atrial fibrillation: would oral anticoagulation have a net clinical benefit for stroke prevention? J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Potze W, Arshad F, Adelmeijer J, et al. Differential in vitro inhibition of thrombin generation by anticoagulant drugs in plasma from patients with cirrhosis. PLoS One. 2014;9:e88390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61:1721–1727. [DOI] [PubMed] [Google Scholar]
- 98.Stangier J, Stähle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008;48:1411–1419. [DOI] [PubMed] [Google Scholar]
- 99.Stangier J, Rathgen K, Stähle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–268. [DOI] [PubMed] [Google Scholar]
- 100.Almegren M. Reversal of direct oral anticoagulants. Vasc Health Risk Manag. 2017;13:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pollack CV, Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377:431–441. [DOI] [PubMed] [Google Scholar]
- 102.Intagliata NM, Maitland H, Pellitier S, et al. Reversal of direct oral anticoagulants for liver transplantation in cirrhosis: a step forward. Liver Transpl. 2017;23:396–397. [DOI] [PubMed] [Google Scholar]
- 103.Intagliata NM, Maitland H, Northup PG, et al. Treating thrombosis in cirrhosis patients with new oral agents: ready or not? Hepatology. 2015;61:738–739. [DOI] [PubMed] [Google Scholar]
- 104.Mendell J, Johnson L, Chen S. An open-label, phase 1 study to evaluate the effects of hepatic impairment on edoxaban pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2015;55:1395–1405. [DOI] [PubMed] [Google Scholar]
- 105.Potze W, Adelmeijer J, Lisman T. Decreased in vitro anticoagulant potency of rivaroxaban and apixaban in plasma from patients with cirrhosis. Hepatology. 2015;61:1435–1436. [DOI] [PubMed] [Google Scholar]
- 106.Agnelli G, Eriksson BI, Cohen AT, et al. Safety assessment of new antithrombotic agents: lessons from the EXTEND study on ximelagatran. Thromb Res. 2009;123:488–497. [DOI] [PubMed] [Google Scholar]
- 107.Caldeira D, Barra M, Santos AT, et al. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart. 2014;100:550–556. [DOI] [PubMed] [Google Scholar]
- 108.Imani F, Motavaf M, Safari S, et al. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014;14:e23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Lédinghen V, Heresbach D, Fourdan O, et al. Anti-inflammatory drugs and variceal bleeding: a case-control study. Gut. 1999;44:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. [DOI] [PubMed] [Google Scholar]
- 111.Russo MW, Pierson J, Narang T, et al. Coronary artery stents and antiplatelet therapy in patients with cirrhosis. J Clin Gastroenterol. 2012;46:339–344. [DOI] [PubMed] [Google Scholar]
- 112.Vivarelli M, La Barba G, Cucchetti A, et al. Can antiplatelet prophylaxis reduce the incidence of hepatic artery thrombosis after liver transplantation? Liver Transpl. 2007;13:651–654. [DOI] [PubMed] [Google Scholar]
- 113.Wolf DC, Freni MA, Boccagni P, et al. Low-dose aspirin therapy is associated with few side effects but does not prevent hepatic artery thrombosis in liver transplant recipients. Liver Transpl Surg. 1997;3:598–603. [DOI] [PubMed] [Google Scholar]
- 114.Vivarelli M, Cucchetti A, La Barba G, et al. Ischemic arterial complications after liver transplantation in the adult: multivariate analysis of risk factors. Arch Surg. 2004;139:1069–1074. [DOI] [PubMed] [Google Scholar]
- 115.Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol. 2017;23:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–60.e1-4. [DOI] [PubMed] [Google Scholar]
- 117.von Köckritz L, De Gottardi A, Trebicka J, et al. Portal vein thrombosis in patients with cirrhosis. Gastroenterol Rep (Oxf). 2017;5:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:82–89. [DOI] [PubMed] [Google Scholar]
- 120.Ageno W, Riva N, Schulman S, et al. Long-term clinical outcomes of splanchnic vein thrombosis: results of an international registry. JAMA Intern Med. 2015;175:1474–1480. [DOI] [PubMed] [Google Scholar]
- 121.Cui SB, Shu RH, Yan SP, et al. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol. 2015;27:914–919. [DOI] [PubMed] [Google Scholar]
- 122.Naeshiro N, Aikata H, Hyogo H, et al. Efficacy and safety of the anticoagulant drug, danaparoid sodium, in the treatment of portal vein thrombosis in patients with liver cirrhosis. Hepatol Res. 2015;45:656–662. [DOI] [PubMed] [Google Scholar]
- 123.Dell'Era A, Iannuzzi F, Fabris FM, et al. Impact of portal vein thrombosis on the efficacy of endoscopic variceal band ligation. Dig Liver Dis. 2014;46:152–156. [DOI] [PubMed] [Google Scholar]
- 124.Werner KT, Sando S, Carey EJ, et al. Portal vein thrombosis in patients with end stage liver disease awaiting liver transplantation: outcome of anticoagulation. Dig Dis Sci. 2013;58:1776–1780. [DOI] [PubMed] [Google Scholar]
- 125.Senzolo M, M Sartori T, Rossetto V, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919–927. [DOI] [PubMed] [Google Scholar]
- 126.Amitrano L, Guardascione MA, Menchise A, et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol. 2010;44:448–451. [DOI] [PubMed] [Google Scholar]
- 127.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. [DOI] [PubMed] [Google Scholar]
- 128.Qi X, De Stefano V, Li H, et al. Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26:23–29. [DOI] [PubMed] [Google Scholar]
- 129.Cerini F, Gonzalez JM, Torres F, et al. Impact of anticoagulation on upper-gastrointestinal bleeding in cirrhosis. A retrospective multicenter study. Hepatology. 2015;62:575–583. [DOI] [PubMed] [Google Scholar]
- 130.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. [DOI] [PubMed] [Google Scholar]
- 131.Levi M, Moore KT, Castillejos CF, et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost. 2014;12:1428–1436. [DOI] [PubMed] [Google Scholar]
- 132.Senzolo M, Tibbals J, Cholongitas E, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharmacol Ther. 2006;23:767–775. [DOI] [PubMed] [Google Scholar]
- 133.Senzolo M, Patch D, Cholongitas E, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without underlying cirrhosis. Cardiovasc Intervent Radiol. 2007;30:545; author reply 546. [DOI] [PubMed] [Google Scholar]
- 134.Van Ha TG, Hodge J, Funaki B, et al. Transjugular intrahepatic portosystemic shunt placement in patients with cirrhosis and concomitant portal vein thrombosis. Cardiovasc Intervent Radiol. 2006;29:785–790. [DOI] [PubMed] [Google Scholar]
- 135.Luca A, Miraglia R, Caruso S, et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut. 2011;60:846–852. [DOI] [PubMed] [Google Scholar]
- 136.Thornburg B, Desai K, Hickey R, et al. Portal vein recanalization and transjugular intrahepatic portosystemic shunt creation for chronic portal vein thrombosis: technical considerations. Tech Vasc Interv Radiol. 2016;19:52–60. [DOI] [PubMed] [Google Scholar]
- 137.Salem R, Vouche M, Baker T, et al. Pretransplant portal vein recanalization-transjugular intrahepatic portosystemic shunt in patients with complete obliterative portal vein thrombosis. Transplantation. 2015;99:2347–2355. [DOI] [PubMed] [Google Scholar]
- 138.Wang Z, Jiang MS, Zhang HL, et al. Is post-TIPS anticoagulation therapy necessary in patients with cirrhosis and portal vein thrombosis? A randomized controlled trial. Radiology. 2016;279:943–951. [DOI] [PubMed] [Google Scholar]
- 139.Aggarwal A, Puri K, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: systematic review. World J Gastroenterol. 2014;20:5737–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walsh KA, Lewis DA, Clifford TM, et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47:333–339. [DOI] [PubMed] [Google Scholar]
- 141.Khoury T, Ayman AR, Cohen J, et al. The complex role of anticoagulation in cirrhosis: an updated review of where we are and where we are going. Digestion. 2016;93:149–159. [DOI] [PubMed] [Google Scholar]
- 142.Vivarelli M, Zanello M, Zanfi C, et al. Prophylaxis for venous thromboembolism after resection of hepatocellular carcinoma on cirrhosis: is it necessary? World J Gastroenterol. 2010;16:2146–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.García-Fuster MJ, Abdilla N, Fabiá MJ, et al. Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig. 2008;100:259–262. [DOI] [PubMed] [Google Scholar]
- 144.Warnaar N, Molenaar IQ, Colquhoun SD, et al. Intraoperative pulmonary embolism and intracardiac thrombosis complicating liver transplantation: a systematic review. J Thromb Haemost. 2008;6:297–302. [DOI] [PubMed] [Google Scholar]
- 145.Boone JD, Sherwani SS, Herborn JC, et al. The successful use of low-dose recombinant tissue plasminogen activator for treatment of intracardiac/pulmonary thrombosis during liver transplantation. Anesth Analg. 2011;112:319–321. [DOI] [PubMed] [Google Scholar]
- 146.Protin C, Bezinover D, Kadry Z, et al. Emergent management of intracardiac thrombosis during liver transplantation. Case Rep Transplant. 2016;2016:6268370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol. 1994;24:1305–1309. [DOI] [PubMed] [Google Scholar]
- 148.Szocik J, Rudich S, Csete M. ECMO resuscitation after massive pulmonary embolism during liver transplantation. Anesthesiology. 2002;97:763–764. [DOI] [PubMed] [Google Scholar]
- 149.Chartier L, Béra J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation. 1999;99:2779–2783. [DOI] [PubMed] [Google Scholar]
- 150.Thabut G, Thabut D, Myers RP, et al. Thrombolytic therapy of pulmonary embolism: a meta-analysis. J Am Coll Cardiol. 2002;40:1660–1667. [DOI] [PubMed] [Google Scholar]
- 151.Feltracco P, Barbieri S, Cillo U, et al. Perioperative thrombotic complications in liver transplantation. World J Gastroenterol. 2015;21:8004–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Algarni AA, Mourad MM, Bramhall SR. Anticoagulation and antiplatelets as prophylaxis for hepatic artery thrombosis after liver transplantation. World J Hepatol. 2015;7:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sugawara Y, Kaneko J, Akamatsu N, et al. Anticoagulant therapy against hepatic artery thrombosis in living donor liver transplantation. Transplant Proc. 2002;34:3325–3326. [DOI] [PubMed] [Google Scholar]
- 154.Hashikura Y, Kawasaki S, Okumura N, et al. Prevention of hepatic artery thrombosis in pediatric liver transplantation. Transplantation. 1995;60:1109–1112. [DOI] [PubMed] [Google Scholar]
- 155.Shay R, Taber D, Pilch N, et al. Early aspirin therapy may reduce hepatic artery thrombosis in liver transplantation. Transplant Proc. 2013;45:330–334. [DOI] [PubMed] [Google Scholar]
- 156.Stine JG, Argo CK, Pelletier SJ, et al. Liver transplant recipients with portal vein thrombosis receiving an organ from a high-risk donor are at an increased risk for graft loss due to hepatic artery thrombosis. Transpl Int. 2016;29:1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]


