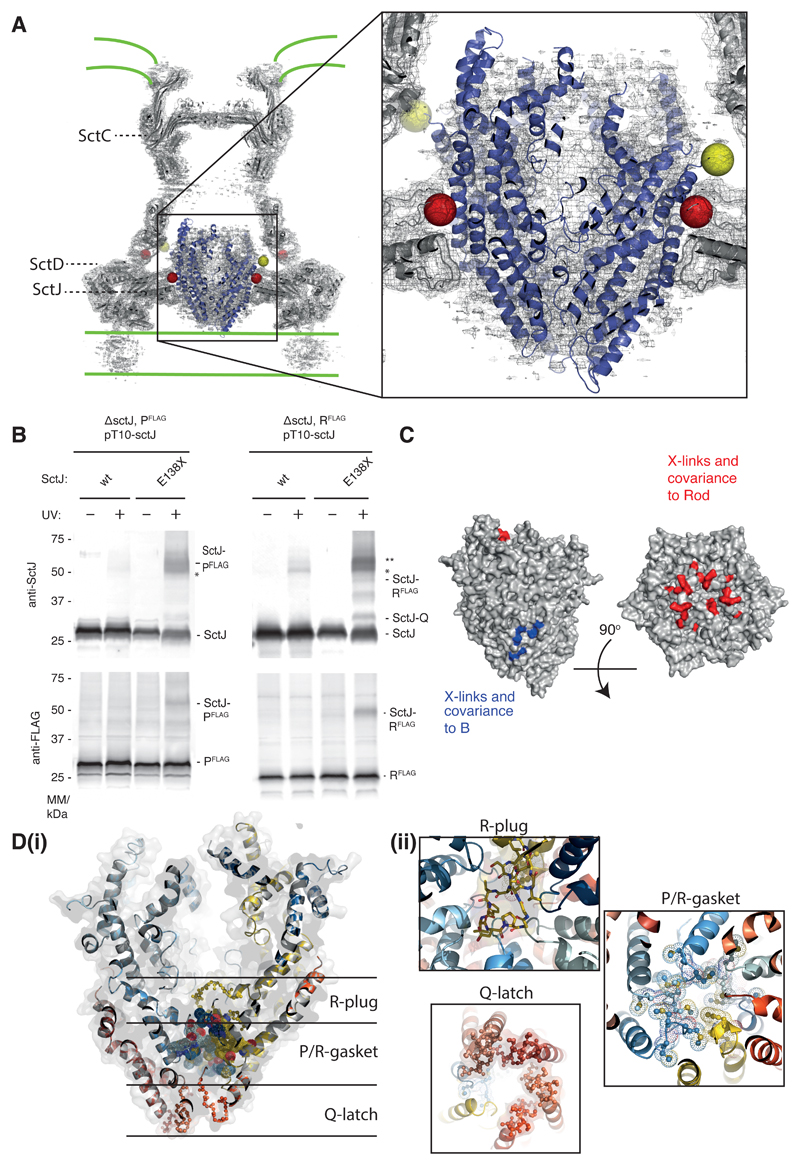
Fig. 4. The P5Q4R1 complex is a core component of the basal body and forms a platform for assembly of the Rod.
(A) Positioning our structure within an earlier high resolution reconstruction of the injectisome basal body 18 (grey surface and cartoon) reveals that the P5Q4R1 complex (blue cartoon) fits the un-occupied density in the center of the basal body. This region of the basal body has previously been called the “cup and socket” and sits above the proposed inner membrane location (shown as green lines). Residues on P that can be cross-linked to the basal body are highlighted by yellow spheres at the Cα position, while residues on SctC and SctJ of the basal body that cross-link to the PQR complex are shown in red. (B) In vivo photo-crosslinking studies reveal cross-links between P5Q4R1 and the inner membrane ring component SctJ in the S. Typhimurium injectisome. The residues involved are highlighted in (A). * SctJ-SctJ pBpa-independent cross-links. ** pBpa-dependent SctJ-ScJ crosslinks result in a crosslink-ladder, of which the homodimeric SctJ-SctJ crosslink is indicated by **. Representative Western blots (upper panel probed with anti-SctJ, lower panel with anti-FLAG tag), n=3. (C) Mapping of earlier cross-linking and our co-variation data (Table S3) onto P5Q4R1 reveal probable binding sites for B (FlhB or SctU) and the (inner) rod components. (D) The export gate is constricted at multiple points (i) a slab section of the entire assembly shows the levels at which the different constriction points operate (ii) views from either above or below each constriction point highlight the structural elements involved.