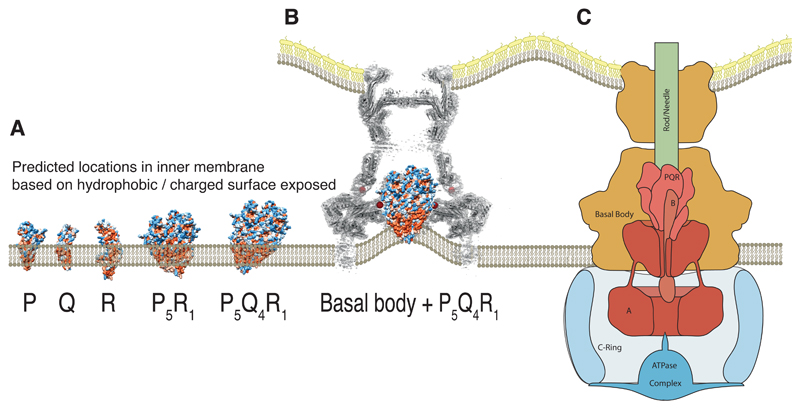
Fig. 6. Placing the complex within the context of the full type three secretion system.
(A) Locating the isolated monomers of P, Q and R within a lipid bilayer is not trivial due to the extended nature of the hydrophobic surface and the large number of charged patches within this surface. However, the assembled object is likely to project into the periplasmic space. (B) In the absence of the other export apparatus components (A and B), earlier tomograms show the inner membrane, within the basal body, is deformed towards the region we now assign to the hydrophobic surface of Q 20. (C) Proposed relative locations of the five export apparatus components within the type three secretion system places the transmembrane portions of the nonameric A at the base of the P5Q4R1 complex. B is likely to form part of the helical export gate complex with the previously assumed “transmembrane” helices driving assembly and with the cytoplasmic domain hanging below a helical P5Q4R1B1 complex.