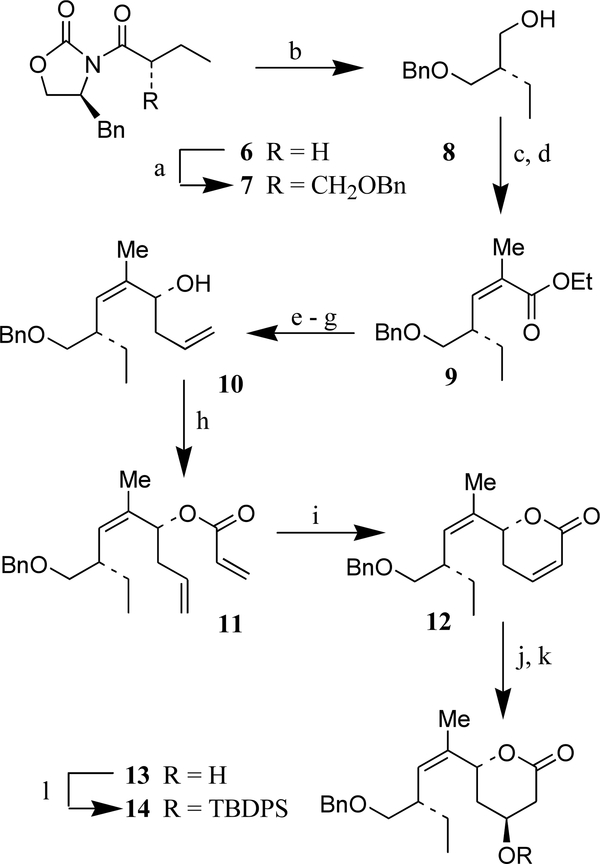
Scheme 1. Reagents and conditions:
(a) TiCl4, Et3N, CH2Cl2, PhCH2OCH2Cl, 0°C, 1.5 h; (b) LiBH4, MeOH, THF, 23°C, 1 h (94%); (c) (COCl)2, DMSO, Et3N, CH2Cl2, −60°C, 45 min; (d) (o-cresol)2PO(CH3)CHCO2Et, NaH, THF, −78 to −20°C, 2 h (90% over two steps); (e) Dibal-H, CH2Cl2, −78° to −40°C, 1 h (96%); (f) Dess–Martin periodinane, NaHCO3, CH2Cl2, 23°C, 1.5 h; (g) CH2CHCH2B[(+)-Ipc]2, Et2O, −80°C, 3 h (65% over two steps); (h) CH2CHCOCl, Et3N, CH2Cl2, 0°C, 2 h (77%); (i) Cl2(Pcy)2RuCHPh, CH2Cl2, 40°C, 12 h (83%); (i) H2O2, 6N-aq NaOH, MeOH, 1.5 h (74%); (k) NaBH4, PhSeSePh, AcOH, iPrOH, 0°C, 30 min (quant.); (l) TBDPSCl, imidazole, DMAP, DMF, 23°C, 13 h (quant.).