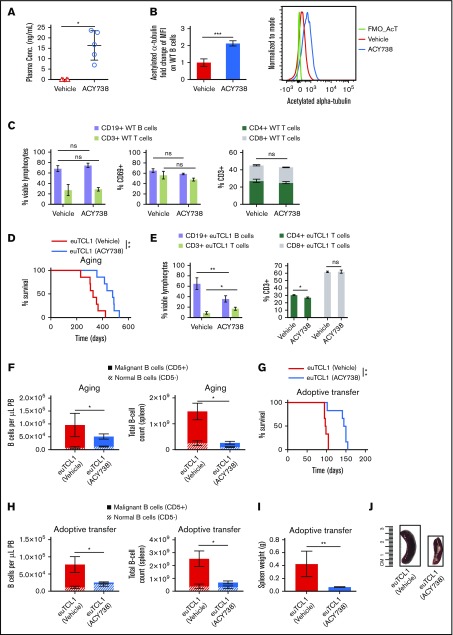
Figure 3.
In vivo activity of selective HDAC6 inhibitor ACY738 in WT and CLL mice. Peripheral blood (PB) and spleens were collected from WT mice after 1 month of being fed vehicle (n = 4) or treated with ACY738 orally at 25 mg/kg (n = 5). (A) Plasma was separated by centrifugation from whole blood, and presence of ACY738 was detected by high-performance liquid chromatography. (B) Acetylation level of α-tubulin was quantified via flow cytometry in PB CD19+ B cells from indicated mice (n = 4 per group). (C) Characterization of immune subsets and activation status from splenocytes (n = 4 per group). (D) Overall survival for euTCL1 aging (n = 7 per group) mice fed vehicle only or treated with ACY738. Data are representative of 3 independent experiments. (E) Characterization of immune subsets from splenocytes derived from aging euTCL1 mice treated with vehicle or ACY738 orally (n = 7 per group). (F) CLL burden was quantified in aging euTCL1 mice fed vehicle only or treated with ACY738 for a duration of 3 months (n = 6 per group). Data were compiled from 2 independent experiments. (G) Overall survival for adoptive transfer euTCL1 mice (n = 6 per group) fed vehicle only or treated with ACY738. Data are representative of 3 independent experiments. (H) CLL burden was also quantified in adoptive transfer CLL mice (n = 8 per group) fed vehicle only or treated with ACY738 for a 12-week duration, and results are representative of 5 independent experiments. (I) Compilation of adoptive transfer CLL mice spleen weights after 12 weeks of indicated treatments (n = 6 per group). (J) Representative spleens from indicated groups. Error bars correspond to standard errors of the mean. *P < .05, **P < .005, ***P < .0005, compared with vehicle-only controls. conc, concentration; MFI, median fluorescence intensity; ns, not significant.