Abstract
BACKGROUND:
Sinonasal malignancies are a rare and heterogeneous group of tumors for which there is a paucity of robust data with which to guide management decisions. The authors used the National Cancer Data Base to better understand the presenting characteristics of these tumors and to compare outcomes by treatment modality.
METHODS:
The National Cancer Data Base was queried for sinonasal malignancies diagnosed between 2004 and 2012. Overall survival was assessed using multivariate analyses and propensity score matching.
RESULTS:
A total of 11,160 patients were identified for the initial analysis. The majority were male, aged 40 to 69 years, with tumors of the nasal cavity or maxillary sinus. Squamous cell histology was most common. The majority of patients presented with advanced tumor stage but without locoregional lymph node or distant metastases. Treatment modalities were compared for squamous cell carcinomas. In multivariate analysis, compared with surgery alone, patients who received adjuvant radiotherapy (hazard ratio [HR], 0.658 [P<.001]), adjuvant chemoradiotherapy (HR, 0.696 [P=.002]), or neoadjuvant therapy (HR, 0.656 [P = .007]) had improved overall survival. Patients who received radiotherapy alone (HR, 1.294 [P=.001]) or chemotherapy alone (HR, 1.834 [P<.001]) had worse outcomes. These findings were validated in propensity score matching. It is important to note that neoadjuvant chemoradiotherapy was associated with achieving a negative surgical margin (odds ratio, 2.641 [P=.045]).
CONCLUSIONS:
Surgery is the mainstay of therapy for patients with sinonasal malignancies, but multimodality therapy is associated with improved overall survival.
Keywords: chemoradiotherapy, head and neck cancer, National Cancer Data Base (NCDB), radiotherapy, sinonasal malignancy
INTRODUCTION
Cancers of the nasal and paranasal sinuses are rare, aggressive tumors that generally are diagnosed at an advanced stage because patients often remain asymptomatic until the tumor becomes large enough to manifest symptoms.1 Although surgery is the mainstay of treatment for patients with these tumors,2 to the best of our knowledge there is limited scientific rationale and weak clinical evidence supporting treatment guidelines in this field. Therapy encompassing a multimodality approach has been shown to improve treatment outcomes.3–8
Although it has been established that maximal safe surgical resection yields the best overall survival (OS) outcomes,2 the role of postoperative radiotherapy is less well defined in the treatment of this disease. Some older series found no survival benefit with adjuvant therapy,9–11 whereas others demonstrated that combined radiotherapy and surgery yield better survival rates.3–7 Older radiotherapy techniques most likely contributed to the lack of therapeutic advantage to chemoradiotherapy, as demonstrated by the high rates of severe long-term toxicities.7 Significant treatment-related toxicity is less common with modern radiotherapy techniques. It is interesting to note that recent advances in radiotherapy have demonstrated significantly improved outcomes with minimal long-term toxicity.3,12–16
The optimal sequencing of chemotherapy and radiotherapy for patients with sinonasal carcinoma remains controversial.17 Small reports have shown a benefit for induction chemotherapy,8,18–22 and numerous institutional and multi-institutional series have shown a benefit for adjuvant therapy.3–6,8,23 To our knowledge, definitive concurrent chemoradiotherapy has been less studied in this disease, with a few series demonstrating low survival outcomes and in-field failures with biologically equivalent doses <65 grays.24 Comparisons of surgery with or without adjuvant chemoradiotherapy reported in retrospective series have emphasized the importance of surgical resection.25 Similarly, other series have shown surgery followed by radiotherapy to be superior to radiotherapy alone.26,27 However, the small cohorts in these retrospective series coupled with heterogeneous histologies significantly limit the broad application of these findings.
In the current study, we used the high number and breadth of cases available in the National Cancer Data Base (NCDB) to investigate outcomes in a modern patient cohort. We specifically examined the presenting characteristics of these tumors according to histology and subsite and compared outcomes by treatment modality for squamous cell carcinomas (SCC). To the best of our knowledge, this is the largest and most comprehensive assessment of sinonasal malignancies reported to date.
MATERIALS AND METHODS
The deidentified NCDB was used for the current study. The NCDB collects data from >1500 community and academic cancer centers and has been reported to represent approximately 70% of all cancer cases in the United States. The NCDB is the result of a collaboration between the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used, or the conclusions drawn from these data by the investigator. The NCDB has established criteria to ensure the data submitted meet specific quality benchmarks. The following NCDB analysis was performed with the approval of our local Institutional Review Board.
We queried the deidentified NCDB file for all patients with primary sinonasal malignancies diagnosed between 2004 and 2012 based on primary site codes C300 to C319 (ICD-0-3). This included the following subsites for initial analysis: maxillary sinus, ethmoid sinus, frontal sinus, sphenoid sinus, nasal cavity, paranasal sinuses, and middle ear. As detailed in Figure 1, our approach to develop a comprehensive and complete cohort for multivariate analysis (MVA) eliminated multiple subsites from our analysis. Ultimately, the subsites included on MVA were maxillary sinus, ethmoid sinus, and nasal cavity. For tumor (T) and lymph node (N) classification, clinical staging information was used primarily when available; otherwise pathologic staging information was applied. Metastases at the time of diagnosis were determined from provided overall stage and “CS mets at diagnosis.” Overall stage was derived from TNM stages using the seventh edition of the AJCC staging manual.28 “Microscopic residual tumor,” “macroscopic residual tumor,” and “residual tumor, NOS [not otherwise specified]” were categorized as positive surgical margins. The additional variables accounted for in the primary analysis included age, sex, race/ethnicity, year of diagnosis, specific disease site, Charlson/Deyo combined comorbidity score (CDCC),29,30 and therapy received. We included the following histologies for our initial analysis of presentation and outcomes: SCC, adenosquamous carcinoma, adenocarcinoma, esthesioneuroblastoma, sinonasal undifferentiated carcinoma (SNUC), adenoid cystic carcinoma (ACC), mucosal melanoma, and mucoepidermoid carcinoma. SNUC was defined by histology code 8020 (“carcinoma, undifferentiated type, NOS”). Sarcomas were excluded from the current analysis. We limited our analysis of outcomes by modality to SCCs. Therapy was categorized into the following groups: surgery alone, radiotherapy alone, chemotherapy alone, definitive chemoradiotherapy, surgery with adjuvant radiotherapy, surgery with adjuvant chemoradiotherapy, and neoadjuvant therapy. The neoadjuvant therapy group was defined to include neoadjuvant chemotherapy, radiotherapy, or chemoradiotherapy because there were limited numbers of patients available for robust comparison from each individual neoadjuvant approach.
Figure 1.
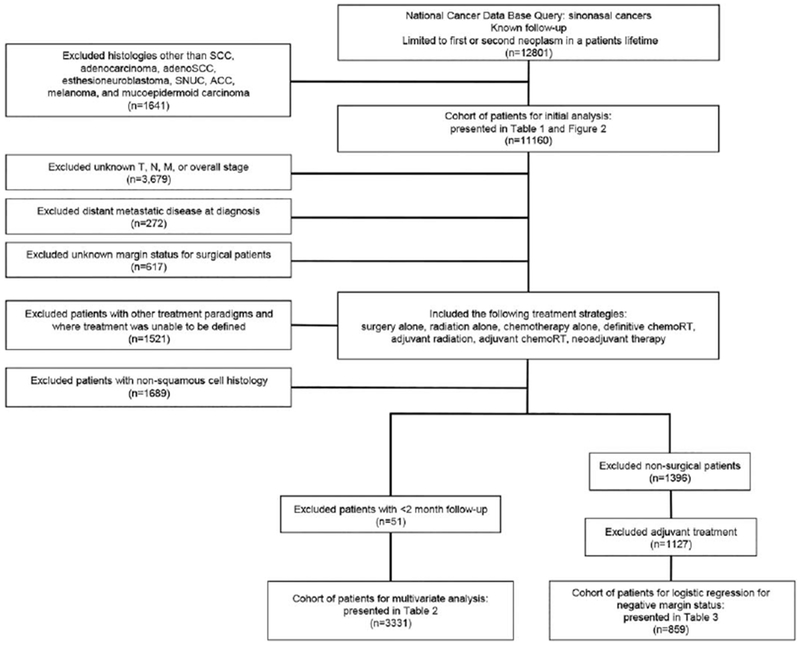
Flowchart illustrating patient cohort selection criteria. ACC indicates adenoid cystic carcinoma; adenoSCC, adenosquamous carcinoma; SCC, squamous cell carcinoma; chemoRT, chemoradiotherapy; SNUC, sinonasal undifferentiated carcinoma.
Surgery was defined as site code 30 or higher. For patients who were coded as receiving “debulking” or “surgery, NOS,” if the surgical margin was negative or microscopic positive, then the patient was coded as having undergone surgery. Ifthe surgical margin was macroscopic positive or unknown, then the patient was excluded because we were unable to determine whether the surgical procedure was oncologic. Concurrent chemotherapy was defined as a chemotherapy start date within 14 days ofthe radiotherapy start date.31 If patients received definitive radiotherapy and received chemotherapy at some point but outside of this 14-day window, they were classified as having received definitive radiotherapy only. Neoadjuvant chemotherapy was defined as starting ≥7 days before surgery. Adjuvant chemotherapy was defined as starting within 30 days after surgery. Patients not meeting either of these criteria were considered to have undergone surgery alone for primary treatment. Ultimately, very few patients were treated with surgery followed by chemotherapy alone and were excluded from analyses. To categorize patients who received neoadjuvant or adjuvant radiotherapy, we first used the information provided under “RX_SUMM_SURGRAD_SEQ.” If this information was not provided, this information then was derived from the days from diagnosis to surgery and the days from diagnosis to radiotherapy.
Our initial query as described above was limited to patients’ first or second lifetime neoplasm. For our initial analysis, we in addition excluded patients with histologies other than those listed above. This yielded 11,160 patients for our primary analysis. To address the impact of differing treatment modalities, we excluded patients with unknown values for predetermined variables, such as staging and surgical margin status. Patients with distant metastatic disease and those with treatment paradigms other than those described above (or for whom we were unable to define the treatment received) were excluded as well. To account for immortal time bias in this retrospective analysis, we also excluded patients with follow-up of <2 months. This yielded 3331 patients for the primary MVA in the current study (Fig. 1).
Statistical Analysis
Statistical analyses were performed using SPSS statistical software (version 23.0; IBM Corporation, Armonk, NY). We performed Kaplan-Meier survival analysis with log-rank comparison. Multivariate Cox regression analysis was performed using OS as the outcome with a significance level of P<.05. The proportional hazards assumption was assessed with a test of Schoenfeld residuals for covariates in all final models as previously described,32 and it returned no significant results. Propensity score matching also was performed for each treatment modality compared with surgery alone. The same variables used in the multivariate analysis were accounted for, including age, sex, CDCC score, race/ ethnicity, year of diagnosis, site, T classification, N classification, facility volume, and surgical margin status. One-to-1 matching without replacement was completed using the nearest neighbor match on the logit of the propensity score for treatment approach with the caliper width set to 0.05 times the standard deviation of the logit of the propensity score, as previously described.33 Univariate cox hazard ratios (HRs) are reported. Logistic regression models were used to assess the association between patient characteristics and treatment. GraphPad Prism (version 5.03; GraphPad Software Inc, La Jolla, Calif) was used for the creation of the Kaplan-Meier curves presented.
RESULTS
We identified 11,160 patients for our primary analysis inclusive of all patients with known follow-up. The majority of patients were aged 40 to 69 years (60.8%); 59.9% were male and 77.9% were non-Hispanic white. SCC was the most represented histology (54.1%). Approximately 16.2% of patients presented with T1 tumors, 9.9% with T2 tumors, 13.8% with T3 tumors, and 31.4% with T4 tumors. Approximately 58.5% of patients had N0 disease and only 5.1% had distant metastases at the time of diagnosis (Table 1). We compared OS by site (Fig. 2A) and histology (Fig. 2B). The median OS by site was 86.2 months for the nasal cavity, 51.2 months for the ethmoid sinus, 35.7 months for the middle ear, 35.2 months for the paranasal sinuses, 29.4 months for the maxillary sinus, 27.6 months for the sphenoid sinus, and 24.8 months for the frontal sinus. The median OS by histology was 98.6 months for adenocarcinoma, 86.1 months for ACC, 52.8 months for SCC, 33.4 months for SNUC, and 22.4 months for melanoma. The median OS was not reached for mucoepidermoid carcinoma, esthesioneuroblastoma, or adenosquamous cell carcinoma histologies.
TABLE 1.
Patient Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age, y | ||
| Birth to 39 | 710 | 6.4 |
| 40-69 | 6781 | 60.8 |
| ≥70 | 3669 | 32.9 |
| Sex | ||
| Male | 6690 | 59.9 |
| Female | 4470 | 40.1 |
| CDCC score | ||
| 0 | 9240 | 82.8 |
| 1 | 1509 | 13.5 |
| 2 | 411 | 3.7 |
| Race/ethnicity | ||
| Non-Hispanic white | 8691 | 77.9 |
| Non-Hispanic African American | 1164 | 10.4 |
| Hispanic | 679 | 6.1 |
| Other/unknown | 626 | 5.6 |
| Y of diagnosis | ||
| 2004-2006 | 3410 | 30.5 |
| 2007-2009 | 3848 | 34.5 |
| 2010-2012 | 3902 | 35.0 |
| Tumor site | ||
| Maxillary sinus | 3398 | 30.4 |
| Ethmoid sinus | 954 | 8.5 |
| Frontal sinus | 110 | 1.0 |
| Sphenoid sinus | 317 | 2.8 |
| Nasal cavity | 5524 | 49.5 |
| Paranasal sinuses | 682 | 6.1 |
| Middle ear | 175 | 1.6 |
| Histology | ||
| Squamous cell carcinoma | 6039 | 54.1 |
| Adenosquamous carcinoma | 104 | 0.9 |
| Adenocarcinoma | 821 | 7.4 |
| Esthesioneuroblastoma | 1113 | 10.0 |
| SNUC | 417 | 3.7 |
| Adenoid cystic carcinoma | 779 | 7.0 |
| Melanoma | 1076 | 9.6 |
| Mucoepidermoid carcinoma | 163 | 1.5 |
| NOS | 648 | 5.8 |
| Tumor classification | ||
| T1 | 1805 | 16.2 |
| T2 | 1104 | 9.9 |
| T3 | 1538 | 13.8 |
| T4 | 3500 | 31.4 |
| Unknown | 3213 | 28.8 |
| Lymph node classification | ||
| N0 | 6531 | 58.5 |
| N1 | 453 | 4.1 |
| N2/N3 | 684 | 6.1 |
| Unknown | 3492 | 31.3 |
| Distant metastases at time of diagnosis | ||
| No | 10045 | 90.0 |
| Yes | 574 | 5.1 |
| Unknown | 541 | 4.8 |
| Overall 7th ed AJCC stage | ||
| I | 1706 | 15.3 |
| II | 923 | 8.3 |
| III | 1465 | 13.1 |
| IVA/IVB | 3473 | 31.1 |
| IVC | 574 | 5.1 |
| Unknown | 3019 | 27.1 |
Abbreviations: AJCC, American Joint Committee on Cancer; CDCC, Charlson/Deyo combined comorbidity score; NOS, not otherwise specified; SNUC, sinonasal undifferentiated carcinoma.
Figure 2.
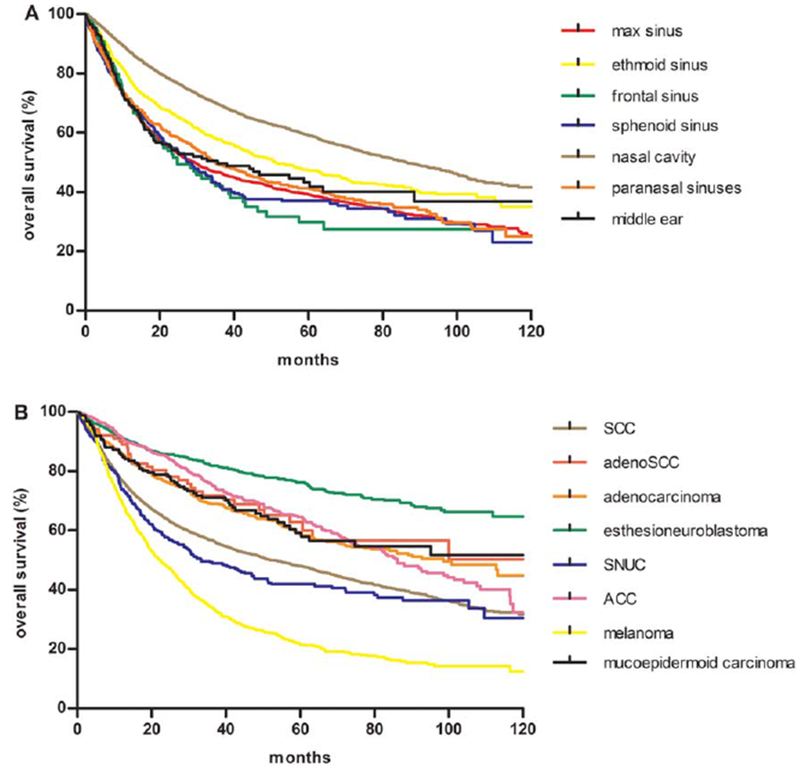
Kaplan-Meier overall survival curves by (A) primary tumor site and (B) histology. ACC indicates adenoid cystic carcinoma; adenoSCC, adenosquamous carcinoma; max, maxillary sinus; SCC, squamous cell carcinoma; SNUC, sinonasal undifferentiated carcinoma.
For comparison of treatment modalities, we limited the current study cohort to patients with SCC histology and included only those patients with known values for the predefined variables for MVA, including TNM and overall stage and surgical margin status. We also excluded patients with metastatic disease at the time of diagnosis and those with treatment paradigms other than those of interest and in cases in which the treatment was unable to be defined. Finally, we excluded patients with <2 months follow-up from the current analysis. Using the above criteria, we identified a total of 3331 patients suitable for analysis. In MVA, we found that age (HR, 1.029 [P<.001]), increasing CDCC score (CDCC of 1: HR, 1.099 [P = .182]; and CDCC of 2: HR, 1.637 [P<.001]), and African American race (vs white: HR, 1.248 [P = .004]) all were associated with worse OS. Hispanic patients had an improved OS (HR, 0.739 [P = .015]) compared with non-Hispanic white patients. By tumor site, patients with nasal cavity tumors were found to have significantly improved OS compared with patients with maxillary sinus tumors (HR, 0.620 [P<.001]); the OS for patients with ethmoid sinus tumors did not differ significantly from that of patients with maxillary sinus tumors. Increasing T classification was associated with worse OS (T2: HR, 1.212 [P = .056]; T3: HR, 1.659 [P<.001]; andT4: HR, 2.035 [P<.001]). Increasing N classification also was associated with worse OS (N1: HR, 1.555 [P<.001]; and N2/N3: HR, 1.600 [P<.001]). Middle tertile-volume facilities had improved outcomes compared with low tertile-volume facilities (HR, 0.844 [P = .006]), and a trend toward improved OS was observed for high tertile-volume centers compared with low tertile-volume centers (HR, 0.888; 95% confidence interval [95% CI], 0.783-1.007 [P = .064]). When comparing treatment modalities, radiotherapy alone (HR, 1.294 [P = .001]) and chemotherapy alone (HR, 1.834 [P<.001]) were associated with worse OS, but patients treated with adjuvant radiotherapy (HR, 0.658 [P<.001]) and adjuvant chemoradiotherapy (HR, 0.696 [P = .002]) had improved outcomes compared with patients treated with single-modality surgery. OS did not differ between definitive chemoradiotherapy and surgery alone (HR, 1.076; 95% CI, 0.899-1.289 [P = .425]). Neoadjuvant therapy, including neoadjuvant chemotherapy, radiotherapy, or chemoradiotherapy, was associated with improved OS (HR, 0.656 [P = .007]) (Table 2). Propensity score-matched analyses with 1-to-1 matching were performed to validate survival outcomes by treatment modality observed in multivariate analyses. Patient characteristics were well balanced between groups (see Supporting Information Table 1). Results were confirmed in propensity score-matched groups with HRs for OS compared with surgery as follows: radiotherapy alone (HR, 1.326; 95% CI, 1.108-1.587 [P = .002]), chemotherapy alone (HR, 1.473; 95% CI, 1.048-2.070 [P = .026]), chemoradiotherapy (HR, 1.136; 95% CI, 0.910-1.417 [P = .260]), adjuvant radiotherapy (HR, 0.762; 95% CI, 0.626-0.929 [P = .007]), adjuvant chemoradiotherapy (HR, 0.689; 95% CI, 0.523-0.909 [P = .008]), and neoadjuvant therapy (HR, 0.681; 95% CI, 0.465-0.996 [P = .048]).
TABLE 2.
Multivariate Cox Regression Analysis for OS Accounting For Relevant Variables In Patients With SCC Histology
| Variable | No. | HR | P | 95% CI |
|---|---|---|---|---|
| Age (continuous variable) | 3331 | 1.029 | <.001 | 1.024-1.033 |
| Sex | ||||
| Male | 2165 | Reference | ||
| Female | 1166 | 0.924 | .140 | 0.831-1.026 |
| CDCC score | ||||
| 0 | 2707 | Reference | ||
| 1 | 477 | 1.099 | .182 | 0.957-1.261 |
| 2 | 147 | 1.637 | <.001 | 1.301-2.061 |
| Race/ethnicity | ||||
| Non-Hispanic white | 2621 | Reference | ||
| Non-Hispanic African American | 376 | 1.248 | .004 | 1.075-1.449 |
| Hispanic | 185 | 0.739 | .015 | 0.579-0.943 |
| Other/unknown | 149 | 1.144 | .271 | 0.900-1.456 |
| Y of diagnosis | ||||
| 2004-2006 | 944 | Reference | ||
| 2007-2009 | 1176 | 0.909 | .118 | 0.806-1.024 |
| 2010-2012 | 1211 | 0.903 | .133 | 0.789-1.032 |
| Tumor site | ||||
| Maxillary sinus | 1579 | Reference | ||
| Ethmoid sinus | 212 | 0.851 | .127 | 0.692-1.047 |
| Nasal cavity | 1540 | 0.620 | <.001 | 0.546-0.705 |
| Tumor classification | ||||
| T1 | 713 | Reference | ||
| T2 | 540 | 1.212 | .056 | 0.995-1.477 |
| T3 | 574 | 1.659 | <.001 | 1.358-2.027 |
| T4 | 1504 | 2.035 | <.001 | 1.700-2.436 |
| Lymph node classification | ||||
| N0 | 2736 | Reference | ||
| N1 | 238 | 1.555 | <.001 | 1.306-1.852 |
| N2/N3 | 357 | 1.600 | <.001 | 1.379-1.855 |
| Facility volume | ||||
| Low | 1054 | Reference | ||
| Mid | 1194 | 0.844 | .006 | 0.748-0.952 |
| High | 1083 | 0.888 | .064 | 0.783-1.007 |
| Surgical margin (for 1959 surgical patients) | ||||
| Negative | 1422 | Reference | ||
| Positive | 537 | 1.575 | <.001 | 1.349-1.839 |
| Therapy | ||||
| Surgery alone | 703 | Reference | ||
| RT alone | 784 | 1.294 | .001 | 1.107-1.511 |
| Chemotherapy alone | 123 | 1.834 | <.001 | 1.421-2.367 |
| Definitive chemoradiotherapy | 465 | 1.076 | .425 | 0.899-1.289 |
| Surgery plus adjuvant RT | 794 | 0.658 | <.001 | 0.553-0.784 |
| Surgery plus adjuvant chemoradiation | 333 | 0.696 | .002 | 0.555-0.874 |
| Neoadjuvant therapy | 129 | 0.656 | .007 | 0.483-0.893 |
Abbreviations: 95% CI, 95% confidence interval; CDCC, Charlson/Deyo combined comorbidity score; HR, hazard ratio; OS, overall survival; RT, radiotherapy; SCC, squamous cell carcinoma.
Finally, we examined factors predictive of achieving a negative surgical margin in patients receiving neoadjuvant therapy. With increasing T classification, patients were less likely to have a negative surgical margin (T2: OR for a negative surgical margin (neg margin), 0.824 [P = .589]; T3: ORneg margin, 0.254 [P<.001]; and T4: ORneg margin, 0.189 [P<.001]). Patients who received neoadjuvant chemotherapy (ORneg margin, 1.388; 95% CI, 0.755-2.553 [P = .291]) or neoadjuvant radiotherapy alone (ORneg margin, 1.496; 95% CI, 0.587-3.810 [P = .399]) were not found to have a statistically significant increased likelihood of achieving a negative surgical margin, but for patients who received neoadjuvant chemoradiotherapy, there was a statistically significant association with negative surgical margin status (ORneg margin. 2.641 [P = .045]) (Table 3).
TABLE 3.
Logistic Regression for Negative Surgical Margins in Patients With SCC Histology Treated With Surgery and Neoadjuvant Therapy
| Variable | OR(−)margin | P | 95% CI |
|---|---|---|---|
| Tumor site | |||
| Maxillary sinus | Reference | ||
| Ethmoid sinus | 0.440 | .015 | 0.228-0.852 |
| Nasal cavity | 1.428 | .104 | 0.929-2.194 |
| Tumor classification | |||
| T1 | Reference | ||
| T2 | 0.824 | .589 | 0.408-1.663 |
| T3 | 0.254 | <.001 | 0.137-0.471 |
| T4 | 0.189 | <.001 | 0.108-0.330 |
| Lymph node classification | |||
| N0 | Reference | ||
| N1 | 0.485 | .038 | 0.245-0.959 |
| N2/N3 | 0.648 | .161 | 0.353-1.188 |
| Therapy | |||
| Surgery alone | Reference | ||
| Neoadjuvant chemotherapy plus surgery | 1.388 | .291 | 0.755-2.553 |
| Neoadjuvant RT plus surgery | 1.496 | .399 | 0.587-3.810 |
| Neoadjuvant chemoradiotherapy plus surgery | 2.641 | .045 | 1.024-6.812 |
Abbreviations: 95% CI, 95% confidence interval; OR(−)margin, odds ratio of having a negative surgical margin; RT, radiotherapy; SCC, squamous cell carcinoma.
DISCUSSION
Sinonasal malignancies present a challenging situation for cancer providers based on their complex anatomic location and the lack of randomized data to guide management. In the current study, we used the NCDB to compile what is to our knowledge the largest study of sinonasal malignancies presented to date. We examined disease prognosis based on histology, subsite, and stage of disease, and assessed outcomes by treatment modality and sequencing for patients with SCC, who comprised the largest subset of patients.
Consistent with published retrospective series, the majority of patients presenting with sinonasal cancer were male, were non-Hispanic white, and had squamous cell histology.5,9 The results of the current study also demonstrated worse outcomes for African American patients and improved outcomes for Hispanic patients compared with non-Hispanic white patients. It is interesting to note that parallel findings have been shown previously in patients with head and neck cancers,34 as well as in patients with non–small cell lung cancer and cervical cancer.35,36
The majority of patients in the NCDB presented with a high T classification but without lymph node metastases, and only approximately 5% of patients had distant metastatic disease at the time of diagnosis. Such a locally aggressive disease emphasizes the importance of improving local therapy. Local disease recurrence has been reported as a predominant treatment failure pattern in the literature.5,17 This also is supported by the findings presented herein that patients with surgically accessible tumors fared better. Patients with tumors of the nasal cavity were found to have far superior OS compared with patients with tumors of other sites. The anatomy of the region, with the proximity of several important structures, adds further complexity to treatment planning. Although both surgical and radiotherapy approaches have advanced in recent years, with endoscopic resections37 and intensity-modulated radiotherapy,38,39 we did not see this translate into an improvement in OS over the time period assessed in MVA in the current study. It is likely that we did not observe an improvement in survival over time in the current study because many of these advancements predate our study period.
A recently reported Surveillance, Epidemiology, and End Results (SEER) analysis exclusive to paranasal sinus squamous cell carcinoma showed similar age and race distributions, although higher rates of lymph node metastases compared with our initial analysis inclusive of additional histologies.40 Patients with mucosal melanoma were found to have the poorest OS, whereas those with esthesioneuroblastoma had the best OS. This finding is consistent with prior studies of mucosal melanoma of the head and neck.41 The 5-year OS rate (approximately 21%) compared similarly with that reported in other studies of mucosal melanoma.42 However, a major confounder in this comparison is the mutational analysis and receipt of targeted therapy and immunotherapy. A major limitation of the NCDB is that neither information regarding driver mutations nor any information concerning the receipt of targeted or immunotherapy is available for analysis.
To the best of our knowledge, the optimal therapeutic protocol for patients with sinonasal cancer remains a controversial entity. Although a multimodality approach has been shown to yield improved survival outcomes in some series,3–8 the optimal combination and sequencing remain unanswered. In concordance with the literature, the results of the current study demonstrate that radiotherapy alone was inferior to surgery alone9 and definitive chemoradiation performed similarly to surgery alone.43 However, patients who received multimodality therapy, in the form of adjuvant radiotherapy, adjuvant chemoradiation, or neoadjuvant therapy, were found to have significantly improved outcomes. This is consistent with some published reports. For example, several series have shown improved survival in patients undergoing surgery and adjuvant radiotherapy or chemoradiotherapy compared with patients receiving radiotherapy alone.6,7,19 Guntinas-Lichius et al demonstrated improved local control and disease-free survival for patients receiving multimodality therapy, including both preoperative and postoperative radiotherapy.26 Another small series presented by Lee et al showed excellent outcomes in a retrospective cohort of 19 patients treated with induction chemotherapy, surgical resection, and adjuvant radiotherapy or chemoradiotherapy.8 In all, the findings from these small series are supported by the findings in the current large cancer registry cohort.
The importance of a negative surgical margin has been shown previously7,9 and was again demonstrated in the current study. Neoadjuvant chemoradiotherapy was associated with an increased likelihood of achieving a negative surgical margin and neoadjuvant therapy (a group inclusive of neoadjuvant chemotherapy, radiotherapy, or chemoradiation) was associated with improved OS. In a small single-institution case series of neoadjuvant chemoradiotherapy for SNUC, 3 patients achieved a pathologic complete response, 2 patients had negative surgical margins, and another 3 patients had close surgical margins,44 thereby supporting the benefit of neoadjuvant chemoradiotherapy for surgical margin status. However, it is interesting to note that another small series of patients with SNUC demonstrated no difference in local control or OS based on surgical margin status.45
As discussed above, the current study supports the use of multimodality therapy for the treatment of these tumors, and we believe the study findings are hypothesis generating. For example, the results herein demonstrate that the addition of radiotherapy to surgery offers an OS benefit either in the neoadjuvant or adjuvant setting, but neither sequence appears to be superior over the other. We suspect that neoadjuvant radiotherapy is preferable in converting patients with unresectable disease to surgical candidates, but outside of that context adjuvant radiotherapy may be preferable given the complexities of operating in a radiated field. Unfortunately, this hypothesis cannot be fully tested in the NCDB due to lack of information regarding initial resectability and surgical complications, but this area of exploration is ripe for prospective assessment. Furthermore, it is not clear that the addition of concurrent chemotherapy to adjuvant radiotherapy further improves OS. This suggests that radiotherapy alone may be sufficient for the sterilization of microscopic disease for SCC in this setting. However, there is a key oncologic outcome that we were unable to assess with the NCDB: freedom from distant metastases. It is possible that chemotherapy improves freedom from distant metastases even if that does not translate into an OS benefit. This may be an important indication for chemotherapy and should be tested prospectively as well.
Overall, to the best of our knowledge, the majority of prior studies regarding this topic consist of small cohorts or case series. Some of these studies failed to demonstrate an advantage to multimodality therapy,9–11 whereas others demonstrated improved survival outcomes, which is consistent with the findings of the current study.3–8 One reason that earlier studies might have failed to demonstrate an improvement with multimodality therapy may be the radiotherapy techniques used, compared with the modern cohort of patients in the current study. Although another more contemporary national cancer registry study also did not demonstrate a benefit to adjuvant radiotherapy,1 it does not appear that this study accounted for other confounding variables. Such outcomes may simply be the result of selection bias because patients with more advanced disease are likely to be offered more extensive therapy. It is important to note that we ultimately may have prospective data to help guide management because there currently are 2 ongoing Italian trials addressing multimodality therapy for sinonasal malignancies (ClinicalTrials.gov NCT02099175 and NCT02099188).46,47 Nevertheless, for now, this large cancer registry cohort offers valuable information to help guide treatment decisions for these difficult cases.
The current study has limitations. It is important to note that the NCDB does not provide data regarding cause-specific survival. Furthermore, because it is a retrospective study, the results of the current study were prone to contamination by other factors for which we could not account. To mitigate as much confounding bias as possible herein, we used MVA and propensity score matching to account for available important variables. Furthermore, to reduce the contribution of immortal time bias, specifically within the context of our neoadjuvant therapy outcomes, we excluded all patients with <2 months of follow-up. Ultimately, we favor this approach but it is important to note that by excluding patients with short follow-up, we also may be selecting for patients who survive aggressive therapies. To better understand the influence of excluding patients with <2 months follow-up, we performed a separate analysis inclusive of all patients and found no significant changes in the results (see Supporting Information Table 2). Another concern is that there is a subset of patients treated nonoperatively, but from the data provided in the NCDB, we cannot know whether these patients presented with resectable or unresectable disease, or the decision-making process that led to them being managed nonoperatively. Despite these limitations, the current study is a large study that asked pointed questions regarding specific treatment modalities to offer cancer providers additional information to help navigate the complex decision making involved in the management ofpatients with these rare diseases.
Conclusions
Herein we present what is to our knowledge the largest study to date of sinonasal malignancies. We demonstrated an advantage of multimodality therapy even when accounting for surgical margin status. Overall, these data emphasize the importance of coordinated multidisciplinary care in the management ofpatients with sinonasal malignancies.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Jones reports grants from Varian Medical Systems, outside the submitted work; In addition, Dr. Jones hasapatent (US Provisional Patent Application 62/368,870, Automated Tracking of Fiducial Marker Clusters in X-Ray Images) pending.
Additional supporting information may be found in the online version of this article
REFERENCES
- 1.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877–885. [DOI] [PubMed] [Google Scholar]
- 2.Resto VA, Chan AW, Deschler DG, Lin DT. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2008;30:222–229. [DOI] [PubMed] [Google Scholar]
- 3.Chen NX, Chen L, Wang JL, et al. A clinical study of multimodal treatment for orbital organ preservation in locally advanced squamous cell carcinoma of the nasal cavity and paranasal sinus. Jpn J Clin Oncol 2016;46:727–734. [DOI] [PubMed] [Google Scholar]
- 4.Corvo R Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol 2007;85:156–170. [DOI] [PubMed] [Google Scholar]
- 5.Danesh-Sani SA, Sarafraz A, Chamani M, Derakhshandeh H. Paranasal sinuses malignancies: a 12-year review of clinical characteristics. Med Oral Patol Oral Cir Bucal 2016;21:e626–e630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen EP, Keus RB, Hilgers FJ, Haas RL, Tan IB, Bartelink H. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys 2000;48:27–35. [DOI] [PubMed] [Google Scholar]
- 7.Le QT, Fu KK, Kaplan M, Terris DJ, Fee WE, Goffinet DR. Treatment of maxillary sinus carcinoma: a comparison of the 1997 and 1977 American Joint Committee on Cancer staging systems. Cancer. 1999;86:1700–1711. [PubMed] [Google Scholar]
- 8.Lee MM, Vokes EE, Rosen A, Witt ME, Weichselbaum RR, Haraf DJ. Multimodality therapy in advanced paranasal sinus carcinoma: superior long-term results. Cancer J Sci Am 1999;5:219–223. [PubMed] [Google Scholar]
- 9.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92: 3012–3029. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JT, Mendenhall WM, Mancuso AA, Cassisi NJ, Million RR. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys 1988;14:11–22. [DOI] [PubMed] [Google Scholar]
- 11.Stern SJ, Goepfert H, Clayman G, et al. Squamous cell carcinoma of the maxillary sinus. Arch Otolaryngol Head Neck Surg 1993;119: 964–969. [DOI] [PubMed] [Google Scholar]
- 12.Askoxylakis V, Hegenbarth P, Timke C, et al. Intensity modulated radiation therapy (IMRT) for sinonasal tumors: a single center longterm clinical analysis. Radiat Oncol 2016;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan R, Bryant C, Li Z, et al. Outcomes of sinonasal cancer treated with proton therapy. Int J Radiat Oncol Biol Phys 2016;95:377–385. [DOI] [PubMed] [Google Scholar]
- 14.Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 2007;67:151–157. [DOI] [PubMed] [Google Scholar]
- 15.Duru Birgi S, Teo M, Dyker KE, Sen M, Prestwich RJ. Definitive and adjuvant radiotherapy for sinonasal squamous cell carcinomas: a single institutional experience. Radiat Oncol 2015;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo AL, Adams JA, Weyman EA, et al. Long-term outcomes after proton beam therapy for sinonasal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2016;95:368–376. [DOI] [PubMed] [Google Scholar]
- 17.Khademi B, Moradi A, Hoseini S, Mohammadianpanah M. Malignant neoplasms of the sinonasal tract: report of 71 patients and literature review and analysis. Oral Maxillofac Surg 2009;13:191–199. [DOI] [PubMed] [Google Scholar]
- 18.Hanna EY, Cardenas AD, DeMonte F, et al. Induction chemotherapy for advanced squamous cell carcinoma of the paranasal sinuses. Arch Otolaryngol Head Neck Surg 2011;137:78–81. [DOI] [PubMed] [Google Scholar]
- 19.Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821–829. [DOI] [PubMed] [Google Scholar]
- 20.Noronha V, Patil VM, Joshi A, et al. Induction chemotherapy in technically unresectable locally advanced carcinoma of maxillary sinus. Chemother Res Pract 2014;2014:487872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ock CY, Keam B, Kim TM, et al. Induction chemotherapy in head and neck squamous cell carcinoma of the paranasal sinus and nasal cavity: a role in organ preservation. Korean J Intern Med 2016;31: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil VM, Joshi A, Noronha V, et al. Neoadjuvant chemotherapy in locally advanced and borderline resectable nonsquamous sinonasal tumors (esthesioneuroblastoma and sinonasal tumor with neuroendocrine differentiation). Int J Surg Oncol 2016;2016:6923730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duthoy W, Boterberg T, Claus F, et al. Postoperative intensitymodulated radiotherapy in sinonasal carcinoma: clinical results in 39 patients. Cancer. 2005;104:71–82. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe BS, Nelson CJ, Gomez DR, et al. Unresectable carcinoma of the paranasal sinuses: outcomes and toxicities. Int J Radiat Oncol Biol Phys 2008;72:763–769. [DOI] [PubMed] [Google Scholar]
- 25.Kang JH, Cho SH, Kim JP, et al. Treatment outcomes between concurrent chemoradiotherapy and combination of surgery, radiotherapy, and/or chemotherapy in stage III and IV maxillary sinus cancer: multi-institutional retrospective analysis. J Oral Maxillofac Surg 2012;70:1717–1723. [DOI] [PubMed] [Google Scholar]
- 26.Guntinas-Lichius O, Kreppel MP, Stuetzer H, Semrau R, Eckel HE, Mueller RP. Single modality and multimodality treatment of nasal and paranasal sinuses cancer: a single institution experience of 229 patients. Eur J Surg Oncol 2007;33:222–228. [DOI] [PubMed] [Google Scholar]
- 27.Mendenhall WM, Amdur RJ, Morris CG, et al. Carcinoma of the nasal cavity and paranasal sinuses. Laryngoscope. 2009;119:899–906. [DOI] [PubMed] [Google Scholar]
- 28.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 31.Amini A, Jasem J, Jones BL, et al. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncol 2016;56:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Amini A, Jones BL, McDermott JD, et al. Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer. 2016;122:1533–1543. [DOI] [PubMed] [Google Scholar]
- 33.Rusthoven CG, Koshy M, Sher DJ, et al. Combined-modality therapy with radiation and chemotherapy for elderly patients with glioblastoma in the temozolomide era: a National Cancer Database analysis. JAMA Neurol 2016;73:821–828. [DOI] [PubMed] [Google Scholar]
- 34.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113:2797–2806. [DOI] [PubMed] [Google Scholar]
- 35.Robin TP, Amini A, Schefter TE, Behbakht K, Fisher CM. Disparities in standard of care treatment and associated survival decrement in patients with locally advanced cervical cancer. Gynecol Oncol 2016;143:319–325. [DOI] [PubMed] [Google Scholar]
- 36.Saeed AM, Toonkel R, Glassberg MK, et al. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival: an analysis of the Survival, Epidemiology, and End Results database. Cancer. 2012;118:4495–4501. [DOI] [PubMed] [Google Scholar]
- 37.Mannu GS, Iyer NG, Shah J. Frontal sinus cancer resection and reconstruction. JRSM Short Rep 2011;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claus F, De Gersem W, De Wagter C, et al. An implementation strategy for IMRT of ethmoid sinus cancer with bilateral sparing of the optic pathways. Int J Radiat Oncol Biol Phys 2001;51:318–331. [DOI] [PubMed] [Google Scholar]
- 39.Madani I, Bonte K, Vakaet L, Boterberg T, De Neve W. Intensitymodulated radiotherapy for sinonasal tumors: Ghent University Hospital update. Int J Radiat Oncol Biol Phys 2009;73:424–432. [DOI] [PubMed] [Google Scholar]
- 40.Ansa B, Goodman M, Ward K, et al. Paranasal sinus squamous cell carcinoma incidence and survival based on Surveillance, Epidemiology, and End Results data, 1973 to 2009. Cancer. 2013;119:2602–2610. [DOI] [PubMed] [Google Scholar]
- 41.Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80:1373–1386. [DOI] [PubMed] [Google Scholar]
- 42.Narasimhan K, Kucuk O, Lin HS, et al. Sinonasal mucosal melanoma: a 13-year experience at a single institution. Skull Base. 2009;19: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold A, Ziglinas P, Ochs K, et al. Therapy options and long-term results of sinonasal malignancies. Oral Oncol 2012;48:1031–1037. [DOI] [PubMed] [Google Scholar]
- 44.Musy PY, Reibel JF, Levine PA. Sinonasal undifferentiated carcinoma: the search for a better outcome. Laryngoscope. 2002;112(8 pt 1): 1450–1455. [DOI] [PubMed] [Google Scholar]
- 45.Chen AM, Daly ME, El-Sayed I, et al. Patterns of failure aftercombined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2008;70:338–343. [DOI] [PubMed] [Google Scholar]
- 46.ClinicalTrials.gov Title. http://clinicaltrials.gov/ct2/show/NCT02099175 and http://clinicaltrials.gov/ct2/show/NCT02099188 Accessed October 2016
- 47.Bossi P, Saba NF, Vermorken JB, et al. The role of systemic therapy in the management of sinonasal cancer: a critical review. Cancer Treat Rev 2015;41:836–843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


