Table 1.
Aldol reaction of ester 2 with representative aldehydes
| Entry | Aldehyde | Compda | Yield (%)b | syn:anti (3/4)c |
|---|---|---|---|---|
| 1 | Me2CHCHO | 3a | 89 | 90:10 |
| 2 | Me2CHCH2CHO | 3b | 93 | 95:5 |
| 3 | trans-PhCH=CHCHO | 3c | 89 | 96:4 |
| 4 | PhCHO | 3d | 94 | 88:12 |
| 5 | Me(CH2)6CHO | 3e | 96 | 82:18 |
| 6 | Ph-C≡C-CHO | 3f | 86 | 36:64d |
| 7 | BnOCH2CHO | 3g | 74 | 99:1 |
| 8 | BnOCH2CH2CHO | 3h | 81 | 99:1 |
| 9 | 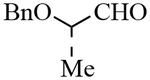 |
3i | 77 | 99:1 |
| 10 | 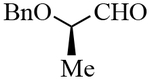 |
3j | 82 | 34:66d |
Major isolated product.
Isolated yield after chromatography.
Ratios determined by 1H MR and HPLC analysis before and after chromatography. Reaction time=1.5–2 h.
Ratio after removal of chiral auxiliary.
