Abstract
Vein graft bypass surgery is a common treatment for occlusive arterial disease; however, long-term success is limited by graft failure due to thrombosis, intimal hyperplasia, and atherosclerosis. The goal of this article is to demonstrate a method for placing bilateral venous interposition grafts in a rabbit, then transducing the grafts with a gene transfer vector that achieves durable transgene expression. The method allows the investigation of the biological roles of genes and their protein products in normal vein graft homeostasis. It also allows the testing of transgenes for the activities that could prevent vein graft failure, e.g., whether the expression of a transgene prevents the neointimal growth, reduces the vascular inflammation, or reduces atherosclerosis in rabbits fed with a high-fat diet. During an initial survival surgery, the segments of right and left external jugular vein are excised and placed bilaterally as reversed end-to-side common carotid artery interposition grafts. During a second survival surgery, performed 28 days later, each of the grafts is isolated from the circulation with vascular clips and the lumens are filled (via an arteriotomy) with a solution containing a helper-dependent adenoviral (HDAd) vector. After a 20-min incubation, the vector solution is aspirated, the arteriotomy is repaired, and flow is restored. The veins are harvested at time points dictated by individual experimental protocols. The 28-day delay between the graft placement and the transduction is necessary to ensure the adaptation of the vein graft to the arterial circulation. This adaptation avoids rapid loss of transgene expression that occurs in vein grafts transduced before or immediately after grafting. The method is unique in its ability to achieve durable, stable transgene expression in grafted veins. Compared to other large animal vein graft models, rabbits have advantages of low cost and easy handling. Compared to rodent vein graft models, rabbits have larger and easier-to-manipulate blood vessels that provide abundant tissue for analysis.
Keywords: Medicine, Issue 139, Animal models of human disease, atherosclerosis, gene therapy, translational studies, rabbit, external jugular vein, common carotid artery, interposition vein graft, vascular disease, adenovirus, helper-dependent adenovirus.
Introduction
Atherosclerosis is a chronic inflammatory disease in which lipid accumulation and inflammation in the blood vessel wall lead to narrowing of the vessel lumen, heart attacks, strokes, and loss of limbs1,2. Percutaneous interventions (e.g., angioplasty and stenting) and medical therapy (e.g., statins and antiplatelet agents) are useful treatments for atherosclerosis; however, they are often ineffective in treating severe obstructive disease both in the coronary and peripheral circulations. Bypass grafting, using autogenous vein segments, remains a common procedure for treating patients with severe, diffuse coronary and peripheral vascular disease3,4. However, vein grafts placed in both the coronary and peripheral circulations have poor long-term patency rates. In the coronary circulation, approximately 10-20% of vein grafts are occluded at 1 year and 50% are occluded by 10 years5,6.In the peripheral circulation, vein graft failure rates are 30-50% at 5 years7.
Gene therapy is an attractive approach for the prevention of vein graft failure because it can deliver a therapeutic gene product precisely at the site of the disease. Accordingly, numerous preclinical studies have tested vein graft gene therapy8,9. However, essentially all of these studies have examined the efficacy at early time points (2-12 weeks)10,11,12,13,14,15,16,17. We are aware of no evidence that gene-therapy interventions can provide durable (years) protection against late vein graft failure that typically results from neointimal hyperplasia and atherosclerosis4. We developed a method that allows durable transgene expression in grafted veins, and thereby allows the testing of gene-therapy interventions at late as well as early time points. To achieve durable transgene expression, the method incorporates HDAd vectors and a delayed transduction strategy. HDAd vectors provide prolonged transgene expression because they lack viral genes, preventing the recognition (and rejection) of transduced cells by the immune system18,19,20,21. Delayed transduction (performed 28 days after the graft placement) prevents the loss of transduced cells during the arterialization process that occurs early after the grafting22.
Other methods that achieve therapeutic transgene expression in the vein graft wall rely on the transduction of the vein graft at the time of the graft placement10,11,12,15,16,17. When measured serially, transgene expression using this approach declines quickly after the transduction22,2,3. Accordingly, studies using this approach have not examined the efficacy beyond 12 weeks after the vein grafting, with most not assessing efficacy beyond 4 weeks. In contrast, our method achieves vein graft transgene expression that persists stably for at least 24 weeks and-based on similar studies performed in arteries-likely continues far longer22,24. We are aware of no other vein graft gene therapy intervention that achieves stable transgene expression of this duration.
We used a rabbit model to develop our method. Others have used rodents, rabbits, or larger animals to test vein graft gene therapy10,11,12,15,16,17,25,26. Compared to rodent models, rabbits are more expensive and are subject to more stringent regulatory requirements. However, compared to larger animals (e.g., pigs and dogs), rabbits are far less expensive to purchase and house and much easier to handle. Moreover, rabbit vessels resemble human vessels physiologically27, they are sufficiently large that they can be used for testing percutaneous interventions28,29, and they provide sufficient tissue that multiple endpoints (e.g., histology, protein, RNA) can be examined using a single blood vessel specimen22,30. In addition, when the rabbits with vein grafts are fed with a high-fat diet, they develop vein graft atherosclerosis31,32, which is a common cause of coronary artery bypass vein graft failure4,5. These atherosclerotic rabbit vein grafts can serve as a substrate for testing gene-therapy interventions delivered with this method. The provided protocol can help investigators to master the technical skills required to achieve durable transgene expression in rabbit vein grafts.
Protocol
All animal protocols and studies were approved by the University of Washington Office of Animal Welfare.
1. Pre-operation (for All Surgeries)
Anesthetize the rabbit with 30 mg/kg ketamine and 1.5 mg/kg xylazine by intramuscular injection (IM) in the paraspinous muscles. NOTE: Food and water are not restricted before surgery.
- While waiting for sufficient depth of anesthesia, set up the tables in the preparation room and operating room (OR).
- Prepare the preparation room for shaving the rabbit's neck and the placement of the intravenous (IV) port in the ear (survival surgery only). Place the ophthalmic ointment, fentanyl patch (survival surgery), hair clippers, alcohol prep pad, 24-G x ¾" catheter, injection port, and surgical tape on the preparation table.
- In the OR, set up the monitoring equipment and associated probes (electrocardiogram (EKG), pulse oximetry (SpO2), and temperature). Prepare the IV pump with a 100 mL saline IV bag with an 18-G or 19-G needle and set the flow rate as 10 mL/h/kg (survival surgery only). Set up the oxygen and isoflurane equipment with a nosecone (vein grafting or harvest surgeries).
- To help secure the nosecone to the rabbit, loop a gauze strip around the tube that supplies gas to the nosecone. This loop should surround the tube where it attaches to the nosecone. The free ends of the gauze strip should both be approximately 45 cm long. Place the nosecone (with attached gauze) on the head end of table.
- Turn on the circulating water and/or forced air warming blanket on the OR table. On the warming blanket, position a rolled towel as a neck support and place the electrocautery dispersive electrode plate.
- As a local analgesic, combine in a syringe 1 mL of 2% lidocaine HCl and 1 mL of 0.5% bupivacaine HCl. Dilute the virus to the desired concentration (here, use 2 x 1011 viral particles/mL for HDAd) in sterile DMEM and keep on ice (gene transfer surgery only).
- After an adequate anesthetic depth is reached, prepare the rabbit for surgery. NOTE: Test for the lack of a pedal withdrawal reflex to ensure adequate anesthetic depth. Continue to monitor the pedal reflex throughout the surgery.
- Apply ophthalmic ointment to the rabbit's eyes.
- To allow for the placement of a rectal temperature probe, press with gloved fingers just above the rectum and move fingers towards the anus to remove stooling from the rabbit's rectum.
- Shave the rabbit from the sternal notch to the edge of mandible. Shave one ear for IV placement and the opposite ear for fentanyl patch administration (survival surgery only). Shave the left rear middle toes for the placement of the SpO2 probe.
- For vector infusion surgery, intubate the rabbit. Use a 4 mm O.D./3 mm I.D. uncuffed endotracheal tube, threaded over an arthroscope. Place the rabbit in sternal recumbency and hyper-extend the neck. Hold the rabbit's mouth wide open and use the arthroscope to visualize the glottis and laryngeal folds. During the inspiration, gently insert the endotracheal tube through the larynx and into the trachea.
- For the survival surgeries, place and secure a 24-G IV catheter into the rabbit's left ear vein and cap it with an injection port. Apply a 25 µg/h fentanyl patch to the rabbit's right ear. NOTE: The protocol is for a surgeon positioned on the rabbit's right side. If the surgeon will be on the rabbit's left side, reverse the sides in this step to keep the wires and IV opposite of the surgeon. Switch the sides in future steps as needed.
- For the vein grafting and harvest surgeries, administer general anesthesia with a nosecone; for the vector infusion surgery, administer general anesthesia via an endotracheal tube. NOTE: Intubation can be used for all surgeries. In our experience, however, the risks of complications from tracheal and laryngeal trauma during intubation can outweigh the benefits of intubation. The surgery that includes gene transfer to a graft (particularly in cholesterol-fed rabbits) is the only surgery in which intubation seems to provide a net benefit.
- Carry the rabbit into the OR. Place the rabbit in a supine position on the operating table with the neck support towel positioned just below the rabbit's head. Gently extend the rabbit's neck until it is straight and approximately horizontal.
- Place the nosecone to the rabbit (vein grafting and harvest surgeries) or connect the endotracheal tube (vector infusion surgery) with O2 at 1 L/min, and start isoflurane. If using a nosecone, secure it by wrapping the ends of the attached gauze strips around the rabbit's neck support towel. Adjust the isoflurane concentration as needed (typically 1-2%) to maintain a surgical level of anesthesia.
- Center the electrocautery dispersive electrode plate under the rabbit's back. Insert the rectal temperature probe and apply the SpO2 probe and EKG leads to the rabbit.
- Connect the saline IV line to the catheter port in the rabbit's left ear and start the IV infusion pump at 10 mL/h/kg. After 1 h, reduce the saline infusion rate to 5 mL/h/kg.
- Loosely tie the rabbit's front legs to the operating table as a restraint. NOTE: Optionally, a small plastic table can be placed over the rabbit to prevent the surgeon from pressing on the rabbit's chest/abdomen, and potentially causing gastroesophageal reflux and aspiration.
- Inject lidocaine/bupivacaine (2 mL, 50/50 mix, step 1.2.4) subcutaneously (SQ) in the neck along the planned incision line, for local anesthesia.
- Have the assistant disinfect the surgical site with 3 alternating scrubs of chlorhexidine gluconate and isopropanol, then spray the site with povidone-iodine. Have the surgeon scrub, gown, and glove, following aseptic principles.
- Perform survival surgeries under aseptic conditions. Have the assistant handle any non-sterile items, and aseptically pass sterile materials to the surgeon or aseptically place them on the draped instrument table.
- Have the surgeon use sterile towels to aseptically manipulate non-sterile equipment such as the microscope. Have the surgeon wear 2x surgical loupes while performing the first half of the surgery.
2. Vein Graft Surgery (Survival)
- Prepare the instruments and sterile field.
- Have the assistant open sterile packs (a table drape, a paper drape, and 6 towels) and aseptically transfer the contents to the surgeon.
- Drape the instrument table. Place the paper drape and sterile towels onto the draped instrument table. With 4 towels, drape the rabbit, leaving only the surgical site on the neck exposed. Lay the paper drape over the rabbit. One towel will be used later, and the last one is a backup.
- Have the assistant open the sterilized instrument pack and aseptically pass the instrument tray to the surgeon. Arrange the instruments on the instrument table.
- Have the assistant open the following equipment and aseptically either pass it to the surgeon or place it onto the instrument table: one 1-mL syringe, one 3-mL syringe, one 20-mL syringe, one 21 G needle, 3-0 polyglycolic acid (PGA) suture, 5-0 PGA suture, 7-0 polypropylene suture, and one 24 G IV catheter.
- Secure the electrocautery cable to the drape covering the rabbit, using a 7.25" Kantrowitz forceps. Drop the plug end of the cable over the edge of the operating table to be connected to the electrosurgery unit and turned on by the assistant.
- Fill a 20-mL syringe with sterile saline from a saline bag or vial held by the assistant. Use this saline as needed to prevent the dehydration of exposed tissue during the operation.
- Prepare 5 mL of heparinized physiologic saline solution by adding 0.1 mL of 5,000 IU/mL heparin to 5 mL of saline solution, i.e., 100 IU heparin/mL, in a small surgical bowl. Use this solution to flush the carotid artery and vein graft regularly during the grafting procedure.
- Cut a hole in the paper drape over the surgical site on the rabbit's neck. Use towel clamps to clamp the corners of the hole in place to the underlying towels.
- Vascular dissection NOTE: Use 2x surgical loupes to aid with this part of the surgery.
- Cut the skin with electrocautery along the midline between the sternal notch and the mandible (approximately 7-9 cm in length) and clamp the rabbit's neck skin to the towels with the towel clamps. At the caudal end, make a short lateral cut through the fascia with electrocautery. Bluntly dissect under the fascia along the entire midline using large scissors. Cut through the dissected fascial layer along the midline with electrocautery.
- Start on the right side. Dissect between the sternohyoid muscle overlying the trachea and the V-shaped sternocephalic muscle, to expose the common carotid artery.
- Carefully dissect a 4-5 cm segment of the common carotid artery and its larger branches (typically 1-2 per side) free from surrounding tissues. Extend the dissection from the base of neck proximally to the crossing of the pharyngeal nerve distally. Use surgical silicon loops to aid in the retraction of the carotid artery during the dissection. Ligate the larger branches of the common carotid artery with 5-0 silk sutures before cutting each branch distally. NOTE: Be careful not to disturb the vagus nerve that parallels the common carotid artery, or the smaller nerves that cross over the artery.
- Repeat the dissection on the left side (step 2.2.3).
- Use tissue-holding forceps to temporarily close the subcutaneous tissue layer. Dissect the superficial fascia from the skin on the right side until the external jugular vein is exposed. Dissect a 4-5 cm segment of external jugular vein and free its branches from the surrounding tissue, ligating small branches with 5-0 silk sutures before cutting the branch.
- Repeat the dissection on the left side (step 2.2.5).
- Inject heparin (150 IU/kg) in the IV catheter and flush with 10 mL of saline.
- Measure a 3-cm segment of external jugular vein on the right side and ligate the caudal end of the segment with 5-0 silk suture. Allow the vein to fill with blood, then ligate the cranial end of the vein segment. Divide the cranial end of the external jugular vein segment, carefully flush the vessel with heparinized normal saline (100 U/mL) using a 3-mL syringe attached to a 24-G IV-catheter. Divide the vein segment at its caudal end. Place the vein segment in a standardized position, in which the caudal and cranial ends are unambiguously identifiable.
- Vein grafting
- Let the assistant remove the surgical loupes from the surgeon and move the surgical microscope (25X) into position. NOTE: The surgeon should drape a sterile towel over the microscope. This allows the surgeon to manipulate the microscope while maintaining sterility.
- Clamp the right common carotid artery at each end of the isolated segment with mini-clamps, placing the cranial clamp first to allow for artery filling, then placing the caudal clamp.
- Cut a 1 × 2 cm piece of stiff paper (available from sterile suture packaging) and place it beneath the common carotid artery, to improve the exposure.
- Perform an arteriotomy just cranial to the caudal clamp (the caudal arteriotomy). The arteriotomy length should be equal to the diameter of the cranial end of the vein segment. Insert the syringe with the IV catheter through the caudal arteriotomy and flush the carotid artery lumen with heparinized normal saline.
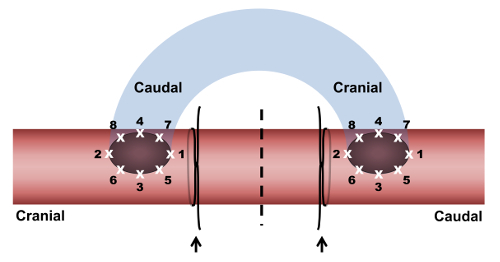
- Suture the cranial end of the vein to the caudal arteriotomy, using 7-0 polypropylene suture with single stitches (Figure 1).
- Place the first stitch to suture the caudal end of the caudal arteriotomy to the cranial end of the vein segment. Place the second stitch 180° from the first stitch, to suture the cranial end of the caudal arteriotomy to the cranial end of the vein segment.
- Place the third stitch at a point equidistant from the first two stitches, on the lateral side of the anastomosis. Place the fourth stitch on the medial side of the anastomosis, equidistant from the first two stitches and across from the third stitch. Place 4 additional stitches (stitches 5-8) between each of stitches 1-4.
- Perform the cranial arteriotomy on the caudal side of the cranial clip. The distance between the cranial end of the cranial arteriotomy and the caudal end of the caudal arteriotomy should be the same as the length of the vein segment. The length of the cranial arteriotomy is equal to the diameter of the caudal end of the vein segment.
- Extend the vein cranially from the anastomosis, laying it on top of the carotid, without introducing any twists. Flush the carotid artery and vein with heparinized normal saline via the cranial arteriotomy. The saline will flow through the artery, into the vein via the caudal anastomosis and will exit from the free and unsutured end of the vein. Flushing the vein also prevents it from twisting.
- Suture the caudal end of the vein to the cranial arteriotomy (Figure 1).
- Place a first stitch to suture the caudal end of the cranial arteriotomy to the caudal end of the vein segment. Place a second stitch to suture the cranial end of the cranial arteriotomy to the caudal end of the vein segment. Complete the anastomosis as described in step 2.3.5.2. Just before the last knot is tied on the cranial anastomosis, briefly open the clamp on the cranial end of the dissected common carotid artery segment, to allow back-bleeding that washes out the air in the carotid artery and grafted vein. Then, tighten the last knot.
- Release the cranial clamp to allow vein graft filling with blood and then release the caudal clamp. Brisk pulsations should be seen in the vein graft. Apply gentle pressure with dry gauze to stop any bleeding at the anastomoses.
- Ligate both ends of the common carotid artery segment that connects the anastomoses with a 3-0 silk suture and divide the carotid halfway between the ligatures (Figure 1). Apply several drops of papaverine (3.5 mg/mL) to the carotid artery adjacent to each anastomosis.
- Inject heparin (75 IU/kg) into the ear IV catheter and flush with 10 mL of saline. At this time, inject buprenorphine (SQ, 0.02 mg/kg) to provide postoperative analgesia until the fentanyl patch has provided analgesic plasma levels of fentanyl. NOTE: An additional buprenorphine injection (SQ, 0.02 mg/kg) may be necessary 6 h after the first injection to maintain analgesia until plasma fentanyl reaches a therapeutic concentration.
- Repeat grafting on the left side (steps 2.2.8, 2.3.2-2.3.9).
- Close the subcutaneous tissue with 5-0 PGA suture using a continuous pattern. Close the skin with 3-0 PGA suture using an intradermal pattern. Bury the knots on both ends.
- Post-operative recovery, cleanup, and care NOTE: Allow the rabbit to recover from anesthesia in a calm, quiet environment. Monitor the rabbit continuously for proper oxygenation and body temperature until it has fully recovered.
- Turn off isoflurane and oxygen and disconnect the anesthesia machine from the rabbit. Disconnect the IV fluid tubing from the rabbit, but leave the IV port in the ear vein for emergent access.
- Transport the rabbit to a recovery cage and place it on its side. Provide thermal support with a warm water blanket (or forced-air warming). Give O2 by nosecone until SpO2 is stable.
- Until the rabbit can sit up and ambulate in its cage, flip the rabbit to its other side every 15 min. Then remove the ear IV cannula and return the rabbit to its cage. Return the rabbit to the company of other animals only after it is fully recovered from anesthesia.
- Dispose of any waste following appropriate protocols for biohazard and sharps waste disposal.
- Postoperatively, check the rabbit's health and surgical wound each day. Administer buprenorphine as need for pain not managed by the fentanyl patch. Remove the fentanyl patch on post-operative day 3.
3. Transcutaneous Ultrasound
- To assess graft patency, perform ultrasound exam on non-anesthetized rabbit 5-7 days after the vein-graft surgery and gene-transfer surgery.
- Wrap the non-anesthetized rabbit securely in a blanket and place the rabbit supine in a veterinary V-trough with its neck extended. Shave the neck, or if needed, remove the hair with a depilatory agent. Apply ultrasound gel to the neck and perform an ultrasound exam. NOTE: An ultrasound exam is also performed on anesthetized rabbits at the time of both the gene transfer surgery and the terminal graft harvest surgery to examine graft patency and to measure graft lumen diameter. The exam is done just before the neck scrubbing and is performed in a similar manner as for the non-anesthetized rabbits.
4. Gene Transfer Surgery Performed ~ 28 Days After the Graft Placement (Survival Surgery)
- Prepare the instruments and the sterile field.
- Follow steps 2.1.1-2.1.3. in vein graft surgery.
- Let the assistant open the following equipment and either aseptically pass it to the surgeon or place it onto the instrument table: six 1-mL syringes (with needle), one 3-mL syringe, one 20-mL syringe, one 21 G needle, one 19-G needle, 3-0 PGA suture, 5-0 PGA suture, 7-0 polypropylene suture, two 24 G IV catheters, and a sterile blood flow probe.
- Follow steps 2.1.5-2.1.6. in vein graft surgery.
- Prepare one 1-mL syringe with 1 mL DMEM for washing the artery and vein graft and prepare two 1-mL- syringes with virus solution (~ 0.5-0.7 mL virus solution/vein graft). Let the assistant thaw the virus and dilute it in DMEM. Prepare one 3-mL syringe with 0.5 mL of lidocaine/bupivacaine (50/50 mix, step 1.2.4).
- Follow step 2.1.8 in vein graft surgery.
- Isolation of vein grafts and adjacent carotid arteries NOTE: 2x surgical loupes should be used for this part.
- Cut the skin with electrocautery along the midline between the sternal notch and the mandible (approximately 7-9 cm in length) and clamp the skin open with towel clamps. Apply 0.5 mL of lidocaine/bupivacaine (50/50 mix, step 1.2.4) to the subcutaneous tissue.
- At the caudal end, make a short lateral cut through the fascia with electrocautery. Bluntly dissect under the fascia along the entire midline. With electrocautery, cut through the dissected fascia along the midline.
- Start on the right side. Dissect between the sternohyoid muscle overlaying the trachea and the V-shaped sternocephalic muscle, to expose the vein graft. Carefully isolate the vein graft and 1.5-2.0 cm of the carotid artery adjacent to the graft on both the cranial and caudal sides.
- Repeat the dissection on the left side following step 4.2.3.
- Measure the blood flow in the vein grafts.
- Fill the surgical wound cavity on the right side with normal saline to enable the transmission of sound waves. Immerse a 2-mm perivascular flow probe (connected to a volume-flow meter) in the saline in the wound cavity and set the flow rate to zero.
- Place the flow probe around the carotid artery caudal or cranial to the vein graft. Record data from the flow probe with an electronic data acquisition system.
- Repeat the flow measurement on the left side following step 4.3.2.
- Calculate the pulsatility based on peak systolic flow rate, minimum diastolic flow rate, and mean flow rate. Also calculate laminar shear stress, based on the flow rate and lumen diameter (measured with transcutaneous ultrasound).
- Infuse vector solution into the vein grafts NOTE: A surgical microscope (16X) is used as needed for the puncturing, infusion, and repair of the carotid artery.
- Have the assistant remove the surgeon's surgical loupes and move the surgical microscope into position. Have the surgeon drape a sterile towel over the microscope. The sterile towel allows the surgeon to manipulate the microscope while maintaining sterility.
- Have the assistant inject heparin (150 IU/kg) in the IV catheter and flush with saline.
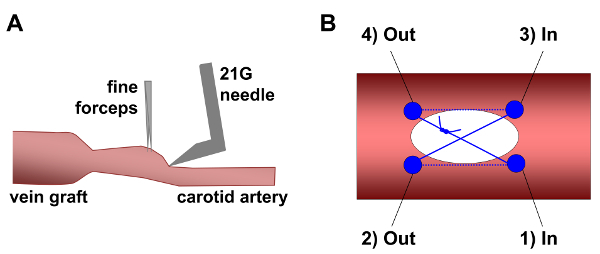
- Use a large needle driver to bend the 21 G needle to approximately 80° just above the bevel (do not bend the bevel; Figure 2A).
- Clamp the common carotid artery at each end of the vein graft with mini-clamps, placing the cranial clamp first to allow for artery filling, then placing the caudal clip. Place the caudal clip approximately 10 mm caudal to the anastomosis, to leave some room for the arteriotomy.
- Put two silk ties around the artery just caudal to the graft and tie a single overhand knot on each - without tightening them.
- Puncture the carotid caudal to the graft with the bent 21 G needle just cranial of the caudal vascular clip. Be careful to not puncture the back or side walls. Advance the needle into the lumen back-and-forth twice to ensure that the lumen is clear, then carefully withdraw the needle. Notes: Common carotid puncture can be aided by grasping the arterial adventitia with fine forceps and gently lifting the front wall while inserting the tip of the needle just caudal to the lift point (Figure 2A). This reduces the risk of hitting the back wall with the needle.
- With several unfolded gauze pads, create a nest to place the syringe used for infusions. Place this gauze nest caudal to the surgical site.
- Put a 24 G IV-catheter on the syringe with DMEM-only and tighten the catheter on the syringe just enough to prevent leakage (the syringe will be removed later in this surgery) and bend the catheter ~ 4 mm from the tip so that the bend holds at about 75° after it is released.
- Insert the IV catheter into the common carotid arteriotomy up to the bend point and wash the vein graft lumen twice with 0.5 mL of DMEM. For each repetition, fill the vein graft with DMEM. Then remove the DMEM from the graft by lightly pressing with a gloved finger at the cranial end of the graft. Carefully slide the finger toward the caudal end of the graft to flush the luminal contents out via the arteriotomy.
- Remove the DMEM syringe from the catheter, leaving the catheter in the vessel. Connect the syringe containing the virus solution, making sure that no air enters the catheter. Move the loosely knotted silk ties down the artery until they are around the catheter tip, but do not tighten them.
- Infuse 0.03 mL of virus solution to push the remaining DMEM out of the catheter. Remove all fluid from the vein graft lumen with a finger, pressing gently from cranial to caudal.
- Tighten the two ties around the artery and catheter tip to seal the lumen. Infuse the virus solution until the vein is distended. Gently lay the syringe down on the nest of gauze. NOTE: It is vital that the vein graft expands to its pre-transduction caliber and remains inflated during the virus infusion. If not, the amount of gene transfer will be significantly decreased.
- Leave the virus-containing solution in the vein graft lumen for 20 min, and then replace the virus-containing syringe attached to the catheter with an empty syringe. Gently aspirate the virus-containing solution from the graft until the vessel collapses. Remove the syringe, leaving the catheter in place. Cut and untie the silk sutures and withdraw the catheter from the vessel. Remove the catheter from the carotid artery carefully to avoid damaging the endothelium.
- With the aid of the surgical microscope, close the arteriotomy with 7-0 polypropylene suture using an X-pattern (Figure 2B).
- Grasp the suture with a needle-holder, enter the vessel at the bottom-right of the arteriotomy, and exit the vessel at the bottom-left. Cross the opening outside the vessel and make the second pass from top-right to top-left.
- Before tightening the suture, briefly release the cranial vascular clip to flush air and residual virus from the vein graft and artery. Blood will flow out of the arteriotomy when the clamp is released.
- Close the arteriotomy by gently pulling the suture ends and tie them together with 2 square knots. NOTE: Pulling the suture too tight will cause bunching of the tissue which will disturb the flow, increase thrombosis risk, and potentially introduce uncontrolled variables.
- Release the cranial vascular clip, then the caudal clip. Stop any bleeding by using gauze to apply light pressure.
- Around this time, inject buprenorphine (SQ, 0.02 mg/kg) to provide postoperative analgesia until the fentanyl patch has provided analgesic fentanyl plasma levels. NOTE: An additional buprenorphine injection (SQ, 0.02 mg/kg) may be necessary 6 h after the first injection to maintain analgesia until plasma fentanyl reaches a therapeutic concentration.
- Repeat the virus infusion protocol on the left side, following steps 4.4.5-4.4.15.
- Wound closure
- Close the subcutaneous tissue with 5-0 PGA suture, using a continuous pattern. Close the skin with 3-0 PGA suture using an intradermal pattern. Bury the knots on both ends.
- Post-operative recovery, cleanup, and care
- Follow step 2.4.
5. Harvest Surgery (Terminal)
- Prepare the instruments and the surgical site.
- Follow steps 2.1.1-2.1.3 in vein graft surgery.
- Let the assistant open and aseptically place on the instrument table (or pass to the surgeon): one 20-mL syringe, one 21G needle, 3-0 silk suture, and a sterile blood flow probe.
- Follow steps 2.1.5-2.1.6 and 2.1.8 vein graft surgery.
- Isolation of common carotid arteries and vein grafts
- Cut the skin with electrocautery along the midline between the sternal notch and the mandible (approximately 7-9 cm in length) and clamp the rabbit's neck skin to the towels with towel clamps.
- At the caudal end, make a short lateral cut through the fascia with electrocautery. Bluntly dissect under the fascia along the entire midline. Cut through the dissected fascia along the midline with electrocautery.
- Start on the right side. Dissect between the sternohyoid muscle overlying the trachea and the V-shaped sternocephalic muscles to expose the common carotid and vein graft.
- Dissect the right vein graft and common carotid artery free from the surrounding tissues. Use surgical silicon loops for the retraction of the artery and the vein graft during the dissection.
- Repeat the dissection on the left side following steps 5.2.3-5.2.4.
Make flow measurements following steps 4.3.1-4.3.3 in gene transfer surgery.
- Harvest of the vein grafts
- With 3-0 silk suture, ligate the right common carotid artery cranial to the graft. Then ligate the carotid caudal to the graft.
- Excise the right vein graft by dividing the adjacent carotid artery between each of the ligations and the adjacent anastomosis. Remove the vein graft from the rabbit and flush the lumen with saline.
- Trim the carotid artery and anastomoses from both ends of the vein graft. Trim away excess adventitial tissue from the vein graft and cut the graft into segments as needed for different endpoint analyses.
- Harvest the left vein graft by repeating steps 5.4.1-5.4.3.
Inject 1 mL of phenytoin/pentobarbital IV to euthanize the rabbit.
Dispose of any waste following appropriate protocols for biohazard and sharps waste disposal.
Representative Results
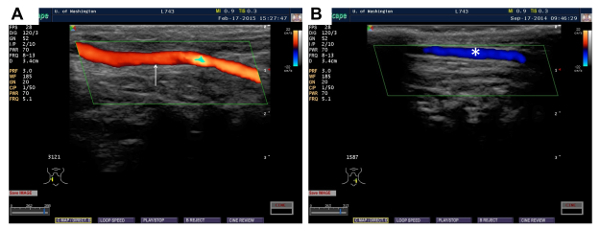
The technical proficiency of a new operator must be validated before the operator can use this method to generate experimental data. The first milestone that a new operator must achieve is consistent vein graft patency after both the initial vein-grafting surgery and the subsequent delayed-transduction surgery. Over 90% patency after each of the surgeries is desirable and achievable. The patency can be assessed non-invasively using transcutaneous ultrasound, which we typically perform on postoperative day 5-7. In the rabbits with patent veins, the ultrasound exam will reveal a large blood vessel with brisk caudal-to-cranial blood flow on both sides of the anterior neck (Figure 3A). If either of the vein grafts is occluded, there will be no detectable caudal-to-cranial blood flow on the side(s) with the occluded graft. However, cranial-to-caudal blood flow will still be detected in the internal jugular vein (Figure 3B).
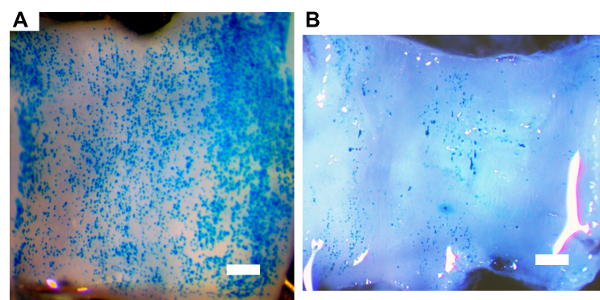
The second milestone that a new operator must achieve is efficient transduction of vein-graft endothelial cells. Here, an adenoviral vector that expresses β-galactosidase is used to evaluate the efficiency of endothelial gene transfer. A new operator in our lab transduced vein grafts with an adenovirus expressing β-galactosidase (AdnLacZ) using the methods described. The grafts were harvested 3 days after the transduction and cut into segments. Some segments were stained with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal). En face imaging of the luminal surfaces showed vein grafts with efficient transduction (Figure 4A) and poor transduction (Figure 4B). To further evaluate the proficiency of a new operator, β-galactosidase mRNA in the extracts of the transduced vein graft is also measured using quantitative reverse transcriptase-mediated PCR and normalizing the signal to the level of glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA measured in the same vein grafts.
The levels of β-galactosidase mRNA in vein grafts transduced by the new operator were not significantly different from the levels of β-galactosidase mRNA in vein grafts transduced by an experienced operator (Figure 5). If mRNA samples from the vein grafts transduced by an experienced operator are not available for comparison, negative control vein graft mRNA can be generated by transducing vein grafts with a non-expressing control vector (AdNull). We have found that the mean levels of β-galactosidase mRNA in vein grafts transduced by an experienced operator are approximately 1,000-fold higher than the background PCR signal for β-galactosidase mRNA measured in AdNull-transduced grafts (Figure 5).
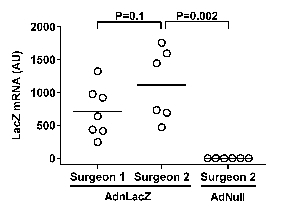
Figure 1. The placement of a reversed right external jugular vein-to-right common carotid artery graft with end-to-side anastomoses. The vein graft (blue) is sutured to the common carotid artery (red) at two anastomoses. At each anastomosis, stitches (white X's) are numbered in the order in which they are placed. For both of the anastomoses, stitch 1 sutures the caudal end of the arteriotomy to the external jugular vein. Stitch 2 sutures the cranial end of the arteriotomy to the external jugular vein. Stitch 3 is placed on the lateral side of the anastomosis at a point equidistant from the first two stitches. Stitch 4 is placed on the medial side of the anastomosis, equidistant from the first two stitches and across from stitch 3. Four additional stitches (stitches 5-8) are placed midway between the four existing stitches. The carotid artery segment between the anastomoses is ligated at both ends with 3-0 silk sutures (arrows) and divided (dashed line) halfway between the ligatures. Left-sided grafts are performed in a similar manner. Please click here to view a larger version of this figure.
Figure 2. The creation and closure of a carotid arteriotomy. (A) The common carotid artery adventitia is grasped near the vein graft with fine forceps and upward traction is applied to the artery wall. This creates a vertical surface, allowing the bent 21 G needle to be inserted approximately parallel to the vessel lumen, decreasing the risk of puncturing the back wall of the artery. (B) The arteriotomy is sutured using 7-0 polypropylene in an X-pattern. The first suture pass enters the artery lumen at the bottom right of the arteriotomy (site 1) and exits the lumen at the bottom left (site 2). The suture then crosses the arteriotomy. The next pass re-enters the lumen at the top right (site 3), and exits the lumen at the top left (site 4). Gentle traction on the ends of the suture (the ends exit from site 1 and site 4) closes the arteriotomy. The suture ends are tied with 2 square knots. The solid blue circles indicate the locations where the suture penetrates the arterial wall. The solid blue lines indicate where the suture passes outside of the arterial wall; dotted blue lines indicate that the suture is located inside the lumen. Please click here to view a larger version of this figure.
Figure 3. Transcutaneous ultrasound images of rabbit necks, evaluating vein graft patency 5-7 days after grafting. (A) Patent vein graft (red) with blood flow in caudal-to-cranial direction is indicated (arrow). (B) Occluded vein graft. No caudal-to-cranial blood flow (which would appear red) is detected. The internal jugular vein (blue) with blood flow in cranial-to-caudal direction is indicated (asterisk). Please click here to view a larger version of this figure.
Figure 4. Beta-galactosidase transgene expression in vein grafts. 28 days after grafting, rabbit vein grafts were transduced by a 20-min incubation of adenoviral vectors expressing β-galactosidase within the lumens of surgically isolated vein grafts. Vein grafts were harvested 3 days after transduction and stained with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside. (A) En face image of the luminal surface of a vein segment showing efficient transduction. (B) En face image of the luminal surface of a vein segment showing poor transduction. Scale bar = 1.0 mm. Please click here to view a larger version of this figure.
Figure 5. Expression of beta-galactosidase (β-gal) transgene mRNA in vein graft segments. 28 days after the placement of jugular vein interposition grafts in rabbit carotids, the grafts were transduced either with an adenoviral vector expressing beta-galactosidase (AdnLacZ) or with a control adenoviral vector that does not express a transgene (AdNull). Surgeries were completed by either an experienced operator (Surgeon 1) or a new operator (Surgeon 2). Vein grafts transduced with AdnLacZ were all harvested 3 days after transduction. RNA extracted from grafts transduced with AdNull and harvested 14 days after transduction was also analyzed, as a negative control. The expression of beta-galactosidase mRNA was quantified by quantitative reverse transcriptase-mediated PCR, with values normalized to glyceraldehyde phosphate dehydrogenase mRNA measured in the same extracts, and expressed as arbitrary units (AU). Bars indicate mean values; p values are from rank-sum tests. Please click here to view a larger version of this figure.
Discussion
Critical steps in this protocol include the management of anesthesia, anticoagulation, surgical manipulation of the artery/grafted vein, and hemodynamic measurements of the grafted vein. Proper management of anesthesia is critical in this multiple survival surgery model that includes two relatively long operations (typically 3-3.5 h for bilateral vein grafting and 1.5-2.5 h for bilateral graft transduction). We have administered anesthesia both via a nosecone and by endotracheal intubation and found that the intubation improved the survival after the graft transduction surgery, possibly because positive pressure ventilation prevents the atelectasis and associated respiratory failure. Rabbits are challenging to intubate, and intubation-related airway trauma can cause post-operative stridor. However, the challenges and complications of the intubation are a small price to pay for the benefit of eliminating most of the perioperative morbidity and mortality associated with the vein grafting surgery.
Anticoagulation is essential in order to prevent the thrombosis of grafted veins, which can occur after either of the survival surgeries. The thrombosis appears to be an early postoperative event, because the vein grafts that are patent on transcutaneous ultrasound examination 5-7 days postoperatively are nearly always patent at harvest. To prevent the thrombosis after the first surgery (i.e., vein grafting), IV heparin is administered before harvesting the first external jugular vein. Based on the plasma half-life of heparin in rabbits (1-2 h), an additional half dose of IV heparin is administered before harvesting the contralateral external jugular vein. In addition, until both arteriovenous anastomoses of an individual vein graft are complete, we flush the lumens of the carotid artery and the vein graft approximately every 8 min with heparinized normal saline. To avoid graft thrombosis, care should be taken not to damage the vein graft-especially the endothelium-during harvesting and grafting. This includes gentle handling of the vein with surgical instruments and leaving a thin layer of adventitial adipose tissue attached to the vein. We also administer a single dose of topical papaverine to the grafted carotid artery, to prevent or reverse vasospasm, which could contribute to thrombosis.
Meticulous attention to surgical technique is required during the vein grafting surgery. To avoid bleeding at the anastomoses, graft thrombosis, and the introduction of uncontrolled hemodynamic variables, the two arteriotomies must be of the proper length. If an arteriotomy is too short, the vein graft ostial wall will be redundant at the site of anastomosis, creating gaps that allow bleeding. If the arteriotomy is too long, stretching the vein to cover the arteriotomy will bring the vein graft walls in close proximity, making it difficult for the surgeon to avoid suturing the opposing vein graft walls together (essentially guaranteeing postoperative thrombosis). Moreover, if the vein graft lumen is narrowed because the vein was stretched to cover an excessively long arteriotomy, a stenosis will be created that increases shear stress, predisposes to thrombosis33, and potentially alters outcome variables such as neointimal growth and vascular remodeling34,35. In addition, it is important to avoid introducing twists into the vein graft, which could result in lumen narrowing or abnormal flow. To help avoid twisting of the graft, we always align the vein segment along the ventral surface of the common carotid artery and ensure there are no twists in the vein. Because of the potential for variable surgical technique to alter vein graft hemodynamics, and because altered hemodynamics can affect outcome variables independently of treatment, we routinely measure blood flow and graft diameter before vector infusion and at the time of harvest. We use these values to calculate shear stress and pulsatility. Vein grafts with hemodynamic measurements outside of predetermined ranges can be excluded objectively from further analysis, decreasing experimental variability and increasing statistical power.
As we developed this method, we made several modifications. Initially, we attempted vein graft gene transfer during the grafting operation. However, under these circumstances, transgene expression was nearly completely lost by 3 days after gene transfer22. Transgene expression was rapidly lost whether the vein segment was transduced in situ and then grafted or whether the vein was grafted first, and then transduced. Only by delaying transduction until after the vein graft had adapted to the arterial circulation (we waited until 28 days after grafting to perform gene transfer, although a shorter delay might be possible), was the rapid loss of transgene expression avoided22. We also converted from nosecone-delivered anesthesia to endotracheal tube-delivered anesthesia for the second (gene transfer) surgery after experiencing intraoperative and postoperative deaths. In addition, after encountering apparent aspiration pneumonia in a postoperative rabbit, we began to place a small plastic table over the rabbit's abdomen (under the sterile drapes). The table was positioned to prevent the surgeon from inadvertently leaning on the rabbit's abdomen, potentially stimulating gastroesophageal reflux and aspiration. We had no more aspiration events after the placement of the table. However, because this placement coincided with the shift to endotracheal intubation, we cannot be sure which intervention is responsible for eliminating aspiration events.
This method also has limitations. In the United States, the use of rabbits instead of rodents necessitates compliance with the requirements of the Laboratory Animal Welfare Act of 1966. Accordingly, investigators using rabbits (or other animals covered by this Act) must have well-equipped operating rooms and expert anesthesiologists and provide a high level of postoperative monitoring and care. The second limitation is that 2 surgeries are required in order to ensure persistent transgene expression22. The second surgery increases the cost of experiments and exposes each rabbit to an additional set of potential operative and perioperative complications. However, we are not aware of any other gene transfer approach that achieves durable transgene expression in vein grafts. The third limitation of this method is that it requires expertise in construction of HDAd vectors and preparation of high-titer HDAd stocks. Many laboratories have expertise with first-generation adenoviral, lentiviral, and adeno-associated viral (AAV) vectors, but relatively few groups have experience with HDAd and would typically need to establish a collaboration to obtain this expertise. The clinical applicability of the method may also be limited. For humans, a second surgery would increase both cost and complication risk. In addition, compared to human saphenous veins (used for coronary and peripheral bypass surgeries), rabbit external jugular veins have very thin walls. It is possible that the wall thickening and remodeling that occurs after grafting a rabbit external jugular vein into the arterial circulation would be attenuated if the vein graft already has a thicker wall. Finally, in experiments in this model lasting up to 6 months, none of the grafted veins developed a stenosis22. Therefore, this method does not appear to model all of the biological processes that contribute to vein graft failure.
In conclusion, the method uses a robust animal model of human vascular disease (rabbits) to generate grafted veins in which transgenes are stably expressed for prolonged periods of time (at least 6 months)22. Stable expression of transgenes in vein grafts will reveal their roles in normal vein graft homeostasis and will clarify their potential for vein graft gene therapy. The method could be improved and made more clinically applicable by the development of techniques that allow percutaneous delivery of vectors to the vein graft, thereby avoiding the second surgery. These techniques might be catheter-based36,37, or they could incorporate specially prepared vectors that are injected into a peripheral vein and then targeted to the vein graft wall, for example by magnetic fields38. The method would also allow testing of vectors other than HDAd for vein graft gene therapy, including lentiviral and AAV vectors as well as non-viral vectors.39,40,41,42
Disclosures
The authors have nothing to disclose.
References
- Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ Res. 2016;118:531–534. doi: 10.1161/CIRCRESAHA.116.308334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes FG, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- Mohr FW, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- de Vries MR, Simons KH, Jukema JW, Braun J, Quax PH. Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol. 2016;13(8):451–470. doi: 10.1038/nrcardio.2016.76. [DOI] [PubMed] [Google Scholar]
- Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013;257:824–833. doi: 10.1097/SLA.0b013e318288c38d. [DOI] [PubMed] [Google Scholar]
- Sabik JF. Understanding saphenous vein graft patency. Circulation. 2011;124(3):273–275. doi: 10.1161/CIRCULATIONAHA.111.039842. [DOI] [PubMed] [Google Scholar]
- Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vasc Med. 2008;13:63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- Robertson KE, McDonald RA, Oldroyd KG, Nicklin SA, Baker AH. Prevention of coronary in-stent restenosis and vein graft failure: does vascular gene therapy have a role. Pharmacol Ther. 2012;136:23–34. doi: 10.1016/j.pharmthera.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Baker AH. Cardiovascular Gene Therapy: Past, Present, and Future. Mol Ther. 2017;25:1095–1106. doi: 10.1016/j.ymthe.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LB, et al. Adenoviral-mediated gene transfer of a constitutively active form of the retinoblastoma gene product attenuates neointimal thickening in experimental vein grafts. J Vasc Surg. 1999. pp. 874–881. [DOI] [PubMed]
- Eefting D, et al. Local lentiviral short hairpin RNA silencing of CCR2 inhibits vein graft thickening in hypercholesterolemic apolipoprotein E3-Leiden mice. J Vasc Surg. 2009;50:152–160. doi: 10.1016/j.jvs.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Handa M, et al. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48(6):1566–1574. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Kloppenburg GT, Grauls GE, Bruggeman CA, Stassen FR. Adenoviral activin A expression prevents vein graft intimal hyperplasia in a rat model. Interact Cardiov Th. 2009;8:31–34. doi: 10.1510/icvts.2008.182329. [DOI] [PubMed] [Google Scholar]
- Eefting D, et al. A novel urokinase receptor-targeted inhibitor for plasmin and matrix metalloproteinases suppresses vein graft disease. Cardiovasc Res. 2010;88:367–375. doi: 10.1093/cvr/cvq203. [DOI] [PubMed] [Google Scholar]
- Eichstaedt HC, et al. Gene transfer of COX-1 improves lumen size and blood flow in carotid bypass grafts. J Surg Res. 2010;161:162–167. doi: 10.1016/j.jss.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Kritz AB, et al. In vivo modulation of Nogo-B attenuates neointima formation. Mol Ther. 2008;16(11):1798–1804. doi: 10.1038/mt.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroulis M, et al. The role of ex-vivo gene therapy of vein grafts with Egr-1 decoy in the suppression of intimal hyperplasia. Eur J Vasc Endovasc. 2010;40:216–223. doi: 10.1016/j.ejvs.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Kochanek S, et al. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and b-galactosidase. Proc Natl Acad Sci U S A. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks RJ, et al. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. P Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-H, et al. Persistence in muscle of an adenoviral vector that lacks all viral genes. P Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- Du L, Zhang J, Clowes AW, Dichek DA. Efficient gene transfer and durable transgene expression in grafted rabbit veins. Hum Gene Ther. 2015;26:47–58. doi: 10.1089/hum.2014.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon KM, et al. Efficient adenoviral gene transfer to early venous bypass grafts: comparison with native vessels. Cardiovasc Res. 1997;35:505–513. doi: 10.1016/s0008-6363(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Flynn R, et al. Expression of apolipoprotein A-I in rabbit carotid endothelium protects against atherosclerosis. Mol Ther. 2011;19:1833–1841. doi: 10.1038/mt.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SJ, et al. Sustained Reduction of Vein Graft Neointima Formation by Ex Vivo TIMP-3 Gene Therapy. Circulation. 2011;124:135–142. doi: 10.1161/CIRCULATIONAHA.110.012732. 11 Suppl. [DOI] [PubMed] [Google Scholar]
- Chiu-Pinheiro CK, et al. Gene transfer to coronary artery bypass conduits. Ann Thorac Surg. 2002;74:1161–1166. doi: 10.1016/s0003-4975(02)03831-6. [DOI] [PubMed] [Google Scholar]
- Byrom MJ, Bannon PG, White GH, Ng MK. Animal models for the assessment of novel vascular conduits. J Vasc Surg. 2010;52:176–195. doi: 10.1016/j.jvs.2009.10.080. [DOI] [PubMed] [Google Scholar]
- Ribichini F, et al. Effects of oral prednisone after stenting in a rabbit model of established atherosclerosis. J Am Coll Cardiol. 2007;50:176–185. doi: 10.1016/j.jacc.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Langheinrich AC, et al. Quantification of in-stent restenosis parameters in rabbits by Micro-CT. Rofo. 2005;177(4):501–506. doi: 10.1055/s-2005-858055. [DOI] [PubMed] [Google Scholar]
- Wacker BK, Dronadula N, Zhang J, Dichek DA. Local Vascular Gene Therapy With Apolipoprotein A-I to Promote Regression of Atherosclerosis. Arterioscler Thromb. 2017;37:316–327. doi: 10.1161/ATVBAHA.116.308258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolak RM, Kirkman TR, Clowes AW. Atherosclerosis in rabbit vein grafts. Arteriosclerosis. 1989;9:374–379. doi: 10.1161/01.atv.9.3.374. [DOI] [PubMed] [Google Scholar]
- Qiang B, et al. Statin therapy prevents expansive remodeling in venous bypass grafts. Atherosclerosis. 2012;223:106–113. doi: 10.1016/j.atherosclerosis.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Casa LDC, Ku DN. Thrombus Formation at High Shear Rates. Annu Rev Biomed Eng. 2017;19:415–433. doi: 10.1146/annurev-bioeng-071516-044539. [DOI] [PubMed] [Google Scholar]
- Chen C, Coyle KA, Hughes JD, Lumsden AB, Ku DN. Reduced blood flow accelerates intimal hyperplasia in endarterectomized canine arteries. Cardiovasc Surg. 1997;5(2):161–168. doi: 10.1016/s0967-2109(96)00086-5. [DOI] [PubMed] [Google Scholar]
- Binns RL, Ku DN, Stewart MT, Ansley JP, Coyle KA. Optimal graft diameter: effect of wall shear stress on vascular healing. J Vasc Surg. 1989;10:326–337. [PubMed] [Google Scholar]
- Oka K, Mullins CE, Kushwaha RS, Leen AM, Chan L. Gene therapy for rhesus monkeys heterozygous for LDL receptor deficiency by balloon catheter hepatic delivery of helper-dependent adenoviral vector. Gene Ther. 2015;22:87–95. doi: 10.1038/gt.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, et al. Prevention of neointimal formation after angioplasty using nuclear factor-kappaB decoy oligodeoxynucleotide-coated balloon catheter in rabbit model. Circ Cardiovasc Interv. 2014;7:787–796. doi: 10.1161/CIRCINTERVENTIONS.114.001522. [DOI] [PubMed] [Google Scholar]
- Chorny M, et al. Site-specific gene delivery to stented arteries using magnetically guided zinc oleate-based nanoparticles loaded with adenoviral vectors. FASEB J. 2013;27:2198–2206. doi: 10.1096/fj.12-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, et al. Three catheter-based strategies for cardiac delivery of therapeutic gelatin microspheres. Gene Ther. 2006;13:1320–1327. doi: 10.1038/sj.gt.3302793. [DOI] [PubMed] [Google Scholar]
- Nouri F, Sadeghpour H, Heidari R, Dehshahri A. Preparation, characterization, and transfection efficiency of low molecular weight polyethylenimine-based nanoparticles for delivery of the plasmid encoding CD200 gene. Int J Nanomed. 2017. pp. 5557–5569. [DOI] [PMC free article] [PubMed]
- Jia SF, et al. Eradication of osteosarcoma lung metastases following intranasal interleukin-12 gene therapy using a nonviral polyethylenimine vector. Cancer Gene Ther. 2002. pp. 260–266. [DOI] [PubMed]
- Morishita R, et al. Intimal hyperplasia after vascular injury is inhibited by antisense cdk 2 kinase oligonucleotides. J Clin Invest. 1994;93:1458–1464. doi: 10.1172/JCI117123. [DOI] [PMC free article] [PubMed] [Google Scholar]


