Abstract
Antibodies, also termed as immunoglobulins (Ig), secreted by differentiated B lymphocytes, plasmablasts/plasma cells, in humoral immunity provide a formidable defense against invading pathogens via diverse mechanisms. One major goal of vaccination is to induce protective antigen-specific antibodies to prevent life-threatening infections. Both thymus-dependent (TD) and thymus-independent (TI) antigens can elicit robust antigen-specific IgM responses and can also induce the production of isotype-switched antibodies (IgG, IgA and IgE) as well as the generation of memory B cells with the help provided by antigen presenting cells (APCs). Here, we describe a protocol to characterize TD and TI Ig isotype responses in mice using enzyme-linked immunosorbent assay (ELISA). In this protocol, TD and TI Ig responses are elicited in mice by intraperitoneal (i.p.) immunization with hapten-conjugated model antigens TNP-KLH (in alum) and TNP-polysaccharide (in PBS), respectively. To induce TD memory response, a booster immunization of TNP-KLH in alum is given at 3 weeks after the first immunization with the same antigen/adjuvant. Mouse sera are harvested at different time points before and after immunization. Total serum Ig levels and TNP-specific antibodies are subsequently quantified using Ig isotype-specific Sandwich and indirect ELISA, respectively. In order to correctly quantify the serum concentration of each Ig isotype, the samples need to be appropriately diluted to fit within the linear range of the standard curves. Using this protocol, we have consistently obtained reliable results with high specificity and sensitivity. When used in combination with other complementary methods such as flow cytometry, in vitro culture of splenic B cells and immunohistochemical staining (IHC), this protocol will allow researchers to gain a comprehensive understanding of antibody responses in a given experimental setting.
Keywords: Immunology and Infection, Issue 139, Thymus-dependent (TD) antigen, thymus-independent (TI) antigen, immunoglobulin (Ig), Ig isotype, memory response, immunization, adjuvant, enzyme-linked immunosorbent assay (ELISA)
Introduction
B lymphocytes are the principal player in humoral immunity and the only cell type in mammals that are capable of producing antibodies, also termed as immunoglobulins (Ig)1,2. Antibodies secreted by B cells provide a formidable defense against invading pathogens via diverse mechanisms including neutralization, opsonization and complement activation, leading to protective immunity3. Secretion of antibodies by B cells is only achieved after full activation of specific B cells, which normally requires two distinct signals3. Signal 1 is relayed by direct binding of the antigen (Ag) to the B cell receptor (BCR) expressed on the surface of specific naïve B cells3. Depending on the source of Signal 2, B cell activation can be divided into thymus-dependent (TD) or thymus-independent (TI)3,4. In a TD antigen response, Signal 2 is provided by activated cognate CD4 T helper (TH) cells, which express CD154, the ligand for the co-stimulatory receptor CD40 expressed on B cells1,2,3. In a TI antigen response, Signal 2 comes from either engagement of Toll-like receptors (TLRs in the case of type 1 TI Ag) or extensive cross-linking of the BCRs (in the case of type 2 TI Ag) on the B cells3,4. Type 1 TI (TI-1) antigens are microbial ligands of TLRs, including bacterial lipopolysaccharides (LPS), viral RNAs, and microbial CpG DNA4,5. Type 2 TI (TI-2) antigens have highly repetitive structure, and are able to deliver prolonged and persistent signaling to the B cell by multiple cross-linking of the BCRs4,6. Typical examples of TI-2 antigens include pneumococcal polysaccharides and hapten-conjugated polysaccharide6,7. Both TD and TI antigens can elicit robust antigen-specific IgM responses and can also induce the production of isotype-switched antibodies (IgG, IgA and IgE) with the help provided by antigen presenting cells (APCs) such as dendritic cells (DCs)1,2,3. Furthermore, both TD and TI antigens are able to induce memory responses with the help of APCs, but TD antigens are more efficient at inducing memory B cell generation3,8.
In this protocol, TD and TI Ig responses are elicited in mice by intraperitoneal (i.p.) immunization with hapten-conjugated model antigens 2,4,6-trinitrophenyl-keyhole limpet hemocyanin (TNP-KLH) and TNP-polysaccharide (neutral, highly branched and high-mass), respectively9,10,11. TD antigens are usually used with an adjuvant to enhance the production of antibodies12. Here in our protocol, TNP-KLH is injected with alum, a commonly used adjuvant in immunization studies12. Other examples of adjuvants that can be used include complete or incomplete Freund's adjuvant (CFA or IFA), monophosphoryl-lipid A/trehalose dicorynomycolate ("Ribi" adjuvant), and CpG oligodeoxynucleotides, etc.13,14. After immunization, mouse sera are harvested at different time points and TNP-specific antibodies in sera are quantified using Ig isotype-specific enzyme-linked immunosorbent assay (ELISA)9,10,11.
ELISA is a plate-based assay that is widely used as a diagnostic tool in medicine and also as an analytical tool in biomedical research15,16. It is used to detect and quantify analytes including antibodies, hormones, cytokines, chemokines, and various antigens, etc. ELISA can be performed in several different formats, including direct, indirect, sandwich and competitive ELISA15,16. In general, it involves the immobilization of the antigen to a solid surface, usually a 96-well microtiter plate, which is incubated with a primary antibody. After incubation, the unbound antibody is washed away. In a direct ELISA, the primary antibody is directly conjugated to an enzyme (typically horseradish peroxidase or alkaline phosphatase), which can cleave a chromogenic substrate to yield a visible color change detected by a signal-detection instrument such as a spectrophotometer15,16. In contrast, if an enzyme-linked secondary antibody is used to bind the primary antibody, then this is considered as an indirect ELISA15,16. Direct ELISA is faster whereas indirect ELISA is more sensitive15,16. In a sandwich ELISA, the plates are coated with a "capture" antibody used to immobilize the antigen of interest in the samples, and then the captured antigen can be detected by another "detection" antibody in a direct or indirect manner15,16. Sandwich ELISA offers high specificity since the antigen is detected by two different antibodies of the antigen. In a competitive ELISA, the competition is established between the sample antigen and the plate-bound antigen for binding to the primary antibody, and then the antigen concentration in sample is quantified by measuring the reduction in signal from the substrate15,16. Competitive ELISA can be performed using the above mentioned direct or indirect format and is useful for the detection of small antigens with only one epitope15,16.
Alternative techniques for the measurement of antibodies include radio-immunoassay (RIA), electrochemiluminescence (ECL) assay and surface plasmon resonance (SPR) assay17. RIA was the first immunoassay developed that measures the presence of an antigen (or antibody) with high specificity and sensitivity using radiolabeled reagents18,19. However, due to the concerns of radioactive toxicity, disposal costs, shelf-life and special licenses to work with radioactive materials, ELISA is a better and more convenient technique for common uses20,21. ECL is a highly sensitive assay in which chemiluminescent reactions are initiated using electricity to generate highly reactive species from stable precursors on the surface of an electrode, and can be used to measure the amount of analytes (such as antigens or antibodies)22. However, ECL requires a special instrument and thus is not as broadly used as ELISA23. SPR is a direct assay that can be used to measure the binding of ligands (e.g., antibodies) to immobilized molecules (e.g., antigens) on a sensor chip surface24. SPR detects the interactions in real time very specifically and does not require the use of labelled reagents as in ELISA. However, SPR also requires a special equipment and has lower sensitivity than ELISA17. Given the limitations of the alternative methods, ELISA is the most suitable and convenient technique for our purpose in this protocol. Here, we describe the use of sandwich ELISA for the analysis of total Ig isotype levels and the procedures of indirect ELISA for the analysis of antigen-specific Ig isotypes.
Protocol
This protocol follows the guidelines of institutional animal research ethics committee of Rutgers University. All mice are used in accordance with NIH guidelines and under an animal protocol approved by the Institutional Animal Care and Use Committee.
1. Preparation of Mice and Collection of Naïve Mouse Sera
Keep all mice for immunization experiments in a specific pathogen-free animal facility.
Use gender-matched, young adult (8–12 weeks old) knockout and littermate control mice that share the same parents and cages for immunization studies.
Plan the immunization and serum collection schedules as depicted in Figure 1.
Harvest naïve serum of each mouse at 7 days (-7 days) before immunization. Follow the retro-orbital bleeding and serum preparation procedures as detailed below in step 4.

2. Preparation of TNP-polysaccharide (a TI Antigen) and TNP-KLH (a TD Antigen)
Dissolve the antigen powders in sterile PBS (pH 7.4) thoroughly to make 0.5 mg/mL of TNP-polysaccharide stock and 1 mg/mL of TNP-KLH stock solutions. Aliquot each antigen stock solution into sterile microfuge tubes at 0.5 mL/tube, and keep the aliquots at -80 °C for long-term storage. Avoid multiple freeze and thaw of the antigen aliquots.
On the injection day, calculate the volume of TNP-polysaccharide (50 µg/100 µL/mouse) or TNP-KLH (100 µg/100 µL/mouse) needed according to the number of mice to be injected. Thaw appropriate number of TNP-polysaccharide or TNP-KLH aliquots at room temperature (RT).
For TNP-KLH injection, first dilute the alum adjuvant (40 mg/mL) with 3 volumes of sterile PBS to a final concentration of 10 mg/mL. Make sure that the alum adjuvant mixture is well-suspended before dilution.
Combine equal volume of 10 mg/mL alum adjuvant slurry from step 2.3 and the thawed TNP-KLH solution (1 mg/mL) into a 5 mL polypropylene tube, mix well, and incubate at 37 °C for 30 min. Prepare this TNP-KLH/alum mix freshly prior to the injection.
3. Immunization of Mice
Perform intraperitoneal (i.p.) injection of TNP-polysaccharide or TNP-KLH/alum to immunize the mice with 1 mL insulin syringes in a biosafety cabinet. Insert the needle at approximately 30° angle preferably in the lower right quadrant of each mouse to prevent injection into the internal organs.
Inject i.p. 100 µL of TNP-polysaccharide per mouse on day 0 for TI Ag immunization (Figure 1A).
Inject i.p. 200 µL of the TNP-KLH/alum mix (freshly prepared in step 2.4) per mouse on day 0 for TD Ag immunization. Repeat the same injection on day 21 as a booster immunization for memory studies (Figure 1B).
After the injection, return each mouse to its cage and keep all the injected mice in specific pathogen-free condition.
4. Retro-orbital Bleeding and Serum Preparation
Harvest mouse sera at different time points: for TNP-polysaccharide immunization, collect mouse sera on day -7 and 7 (Figure 1A); for TNP-KLH/alum immunization, collect mouse sera on day -7, 7, 14 and 28 (Figure 1B).
For Retro-orbital bleeding, anesthetize each mouse with 5% isoflurane for 1–2 min in a biosafety cabinet25. Perform pedal reflex to ensure adequate anesthesia of each mouse.
Hold the anesthetized mouse in one hand with the forefinger and thumb pulling the skin around the eyeball back so that the eyeball protrudes out of the socket25. Insert a non-heparinized Pasteur pipette or capillary tube at an angle of 45° into the inner corner of the eye socket underneath the eyeball25.
Apply gentle downward pressure and rotate the pipette or tube gently to break into the vein and collect 150–200 µL of blood in the pipette or tube. Immediately transfer the blood to a sterile 1.5 mL microfuge tube and return the mouse to its cage to recover.
Let the blood samples sit at RT for 1–2 h to coagulate.
Centrifuge the coagulated blood samples at 13,000 x g for 10 min at 4 °C. Transfer the clear serum on top of the blood clot to a new sterile 1.5 mL microfuge tube.
Repeat step 4.6 one more time to remove residual blood clot and collect the clear serum.
Aliquot the serum into sterile 1.5 mL microfuge tubes at 50 µL/tube, and store the sera at -80 °C.
Alternate the eye for bleeding at different time points so that each eye is bled at most twice for the whole experiment. At the terminal bleeding, collect up to 1 mL of blood from each mouse before euthanasia. Euthanize the mouse with 5% CO2 followed by cervical dislocation. NOTE: Splenic B cells can be harvested for flow cytometric analyses and in vitro culture studies. We also prepare genomic DNA from splenocytes to verify the genotype of each mouse.
5. Mouse Ig Isotype-specific ELISA
- Prepare the buffers and solutions before ELISA.
- Prepare 500 mL of Coupling Buffer (PBS, pH 7.4).
- Prepare 500 mL of Wash Solution (PBS-T (0.05% Tween 20), pH 7.4).
- Prepare 100 mL of Blocking Buffer (1% BSA in PBS, pH 7.4, stored at 4 °C).
- Prepare 1 L of Substrate Buffer (1 M diethanolamine, pH 9.8 (97 mL of diethanolamine in 1 L of H2O, pH adjusted using 10 M HCl) and 0.5 mM MgCl2). Protect the Substrate Buffer from light by wrapping the bottle with aluminum foil. Store at 4 °C.
- Prepare 100 mL of Stop solution (3 M NaOH).
- Coat the ELISA plates:
- For total serum Ig isotype ELISA, coat 96-well immuno plates with 10 µg/mL of isotype-specific capturing polyclonal goat anti-mouse Ig (M, G1, G2a, G2b, G3, A, or E) Abs in Coupling Buffer (PBS) at 100 µL/well.
- For TNP-specific Ig isotype ELISA, coat 96-well immuno plates with 10 µg/mL of TNP(38)-BSA in PBS at 100 µL/well.
- For high affinity TNP-specific Ig isotype ELISA, coat 96-well immuno plates with 10 µg/mL of TNP(3)-BSA in PBS at 100 µL/well26. Incubate the plates at 4 °C overnight.
After coating incubation, wash the plates 2 times with 200 µL/well of PBS-T. Discard the Wash Buffer and blot dry the plates (tap each plate upside down on a stack of paper towels) after each wash.
Block the plates: add 200 µL/well of Blocking Buffer (1% BSA in PBS) into the coated plates, and incubate the plates for 1 h at RT.
- While the plates are blocking, prepare dilutions of Ig isotype standards and serum samples in Blocking Buffer in a separate, untreated 96-well plate at 150 µL/well.
- Prepare 250 ng/mL Ig isotype standards as the starting standard concentration (St01) and make 7 to 10 of 1:2 serial dilutions of the Ig standards (St02 to St07 or St10, Figure 2A).
- Prepare mouse serum samples at a 1:100 or 1:500 dilution factor as the starting dilution and make 3 or 4 of 1:10 serial dilutions for total Ig isotype or 1:5 serial dilutions for TNP-specific Ig isotype of each serum sample (Figure 2A). For IgE ELISA, prepare mouse serum samples at a 1:2 dilution factor as the starting dilution, and make 3 of 1:5 serial dilutions of each serum sample.
After blocking, wash the plates from step 5.4 three times with 200 µL/well of PBS-T and blot dry the plates after each wash.
Transfer 100 µL/well of diluted Ig isotype standards (appropriate for the capture and detection Abs) and diluted serum samples from step 5.5 to the plates prepared in step 5.6. Incubate the plates with the standards and samples at 4 °C overnight.
Wash the plates 3 times with 200 µL/well of PBS-T and blot dry the plates after each wash.
In each plate, add 100 µL/well of 10 µg/mL of an appropriate alkaline phosphatase (AP)-conjugated isotype-specific goat anti-mouse Ig (M, G1, G2a, G2b, G3, A, or E) Abs diluted in Blocking Buffer.
Incubate the plates with AP-conjugated Abs for 1 - 2 h at RT. For IgE detection, incubate the plates for 1–2 h at 37 °C.
Prepare 1 mg/mL of Substrate Solution by dissolving two tablets of 5 mg phosphatase substrate in 10 mL of Substrate Buffer.
Wash each plate 5 times with 200 µL/well of PBS-T and blot dry the plates after each wash.
Add 100 µL/well of Substrate Solution into each plate. Allow the reaction to develop at RT, which usually takes only a few minutes.
- Read each plate at 405 nm using a microplate reader with its associated software.
- Click "Settings" to set the wavelengths "Lm1" at "405 nm" and click "OK".
- Click "Template" to assign "Blank" wells, "Standards" wells and "Unknown" wells according to the 96-well plate setup.
- For "Standards" wells, click "Series" to set the "First Sample" as "St01", select "Start From" at "Top", and set "Replicates" as "2". Check "Sample Descriptor" and input "Standard Value": select "Units" as "ng/mL", set "Starting value" as "250", set "Step by" as "2". Click "OK".
- Click "Read" to read the plate when the most concentrated standard reaches an optical density (OD) of ~1.
- Click the "File" menu and click "Save" to save the file.
- Click "Read" to read the plate again when the most concentrated standard approaches OD405 of ~1.5, 2, and 2.5, respectively.
Stop the reaction by adding 25 µL/well of 3M NaOH. Complete steps 5.12 to 5.15 for one plate at a time.
- Analyze ELISA data using the software associated with the microplate reader:
- Check the OD405 values of the Ig isotype standards of read files and select an appropriate reading with good linear range of the standards for detailed data analysis.
- Select the 4-Parameter fitting program to plot standard curves. Check the co-efficient of the standard curve (R2), which is required to be > 0.98 to ensure good data quality.
- Check whether the OD405 values of each sample decrease with increasing dilution factors. Retrieve the concentration of all diluted sample wells by clicking "Unknowns".
- Select an appropriate well of each sample with OD405 in the linear range of the Ig isotype standard curve for concentration calculation.
- Calculate the serum Ig isotype concentration of each sample using the formula: serum Ig concentration = selected well concentration x dilution factor.
Plot graphs and perform statistical analyses using an appropriate software9,10,11. Make vertical scatter plots of serum Ig isotype levels to compare the Ig responses at the same time point. For TD memory response studies, make the time-course response curves to examine the Ig isotype responses before and after the booster immunization. Use error bars to show standard deviation (SD) of each group of samples. To compare Ig isotype responses between two genotypes of mice, use the unpaired t test for two-tailed data to determine the statistical significance. Set the p value < 0.05 as significantly different.
Representative Results
We have used this protocol to investigate the roles of a critical regulator of the immune system, TRAF3, in TI and TD Ig isotype responses9,10,11. TRAF3 directly or indirectly regulates the signal transduction of a number of innate and adaptive immune receptors, including the TNF receptor superfamily, Toll-like receptors and T cell receptor/CD28, among others27,28. We hypothesize that TRAF3 plays distinct roles in different immune cell subsets to regulate antibody responses. To test this hypothesis, we determined TI and TD Ig isotype responses using conditional TRAF3 knockout mice that have the Traf3 gene specifically deleted in B cells, T cells, or myeloid cells, respectively9,10,11. Representative immunization and serum collection schedules for TI and TD Ig studies are depicted in Figure 1. Representative IgG1 and IgG2b ELISA results are shown in Figure 2 and Figure 3 to illustrate how ELISA works. These include the plate setup of diluted standards and samples (Figure 2A), an image of the plate after the addition of the AP substrate (Figure 2B), the read results of OD405 (Figure 2C, 2D), the values of standard dilutions (Figure 2E, 2F), the standard curves (Figure 3A, 3B), the values of diluted samples (Figure 3C, 3D), and the calculation of serum IgG1 and IgG2b concentrations in the samples (Figure 3E, 3F). Figure 4 shows representative results of total Ig isotypes in sera of naïve mice. We demonstrated statistically increased basal serum levels of IgM, IgG2a, IgG2b, IgG3 and IgA in B cell-specific TRAF3-/- (B-TRAF3-/-) mice as compared to gender- and age-matched TRAF3-sufficient littermate control mice (LMC). This hyperglobulinemia of B-TRAF3-/- mice is caused by the expanded B cell compartment in peripheral lymphoid organs due to prolonged survival of mature TRAF3-/- B cells9. Figure 5 shows the representative results of TI and TD Ig isotype responses of mice to immunization with TNP-polysaccharide and TNP-KLH, respectively. These results revealed a significantly higher TI, TNP-specific IgG3 level and also elevated TD, TNP-specific IgG2b levels in myeloid cell-specific TRAF3-/- (M-TRAF3-/-) mice than in LMC. Such increased TI IgG3 and TD IgG2b responses observed in M-TRAF3-/- mice are likely due to increased production of the pro-inflammatory cytokines IL-6 and IL-12 by TRAF3-/- macrophages and DCs following immunization11. Figure 6 shows the representative results of TD primary and memory responses of mice to TNP-KLH immunization. These results demonstrated partially decreased TD IgM primary response and defective IgG1 primary and memory responses in T cell-specific TRAF3-/- (T-TRAF3-/-) mice. The defective TD primary and memory responses of T-TRAF3-/- mice result from impaired activation of TRAF3-/- CD4 T cells upon T cell receptor and CD28 co-engagement10. Taken together, the protocol described in this article allowed us to delineate the specific roles of TRAF3 in different immune cell subsets in regulating TI and TD Ig isotype responses in mice.
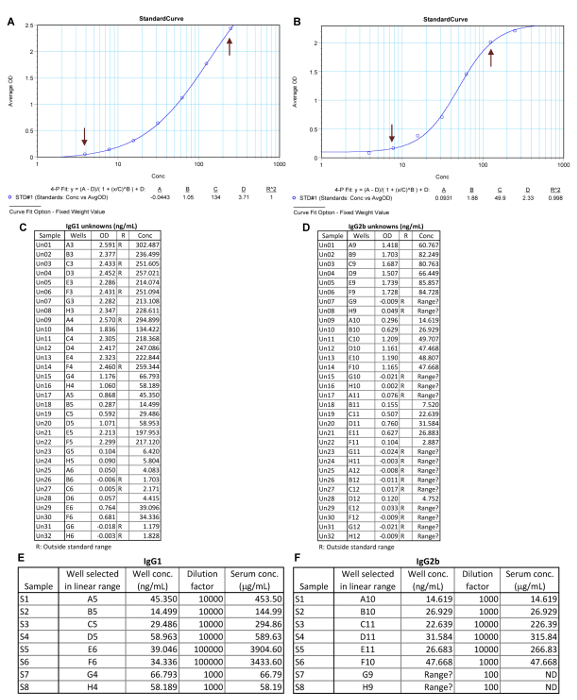
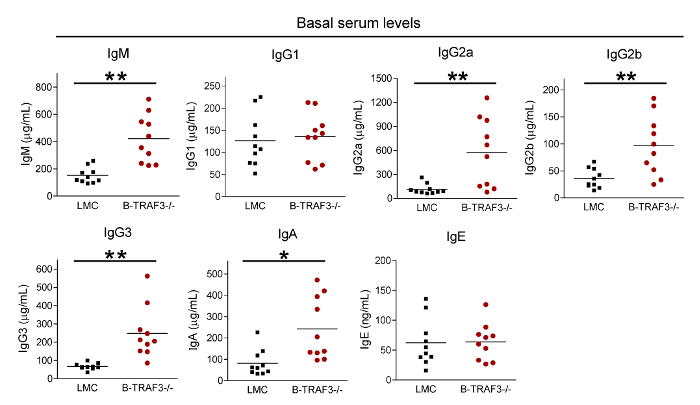
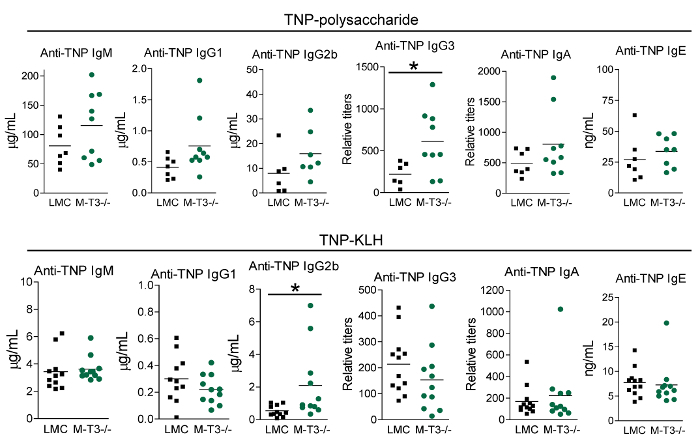
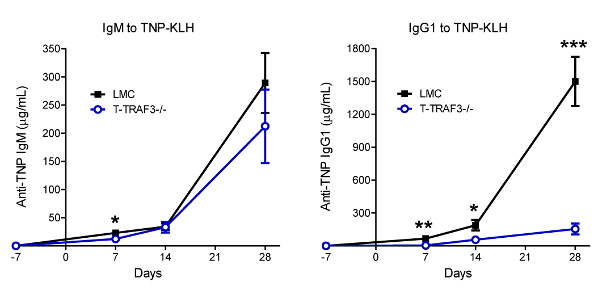
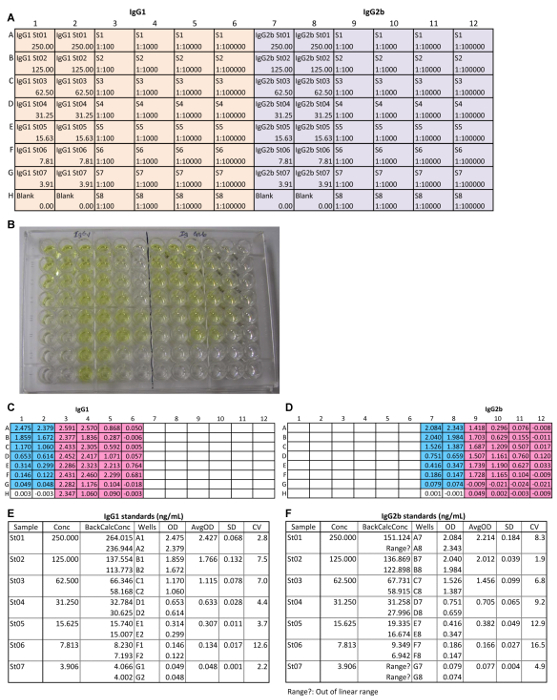
Figure 1: Typical TI and TD Ag immunization and serum collection schedules. (A) TNP-polysaccharide experiments. (B) TNP-KLH experiments. Please click here to view a larger version of this figure.
Figure 2: Representative IgG1 and IgG2b ELISA. (A) The 96-well plate setup includes the wells of blank, 7 serial dilutions (1:2) of mouse IgG1 and IgG2b standards (St01 to St07), and 4 serial dilutions (at 1:10) of the 8 mouse serum samples (S1 to S8). The concentration of standards is given at the bottom of each standard well. The dilution factor of the samples is given at the bottom of each sample well. (B) An image of the plate at 5 min after the addition of the AP substrate. The plate read results of IgG1 (C) and IgG2b (D) at 405 nm. The values of OD405 and concentrations of different dilutions of mouse IgG1 (E) and mouse IgG2b (F) standards. Conc, concentration; BackCalcConc, back calculated concentration; OD, optical density; AvgOD, average OD of the replicates; SD, standard deviation; CV, coefficient of variation. Please click here to view a larger version of this figure.
Figure 3: Representative IgG1 and IgG2b ELISA data analysis. The standard curves of IgG1 (A) and IgG2b (B). The co-efficient R2 is > 0.98 in both standard curves. Arrows indicate the linear range of the standard curves. Values of OD405 and concentrations of IgG1 (C) and IgG2b (D) in Unknowns (diluted serum samples). R, range; Conc, concentration. Calculation of mouse serum concentrations of IgG1 (E) and mouse IgG2b (F) for the 8 samples. ND, not detectable by this ELISA. Please click here to view a larger version of this figure.
Figure 4: Representative results of total Ig isotypes in sera of naïve mice. Sera were collected from gender-matched, 10-12 weeks old naive LMC and B-TRAF3-/- mice (n = 10 for each genotype; genetic background: 129xC57BL/6). Basal serum levels of total IgM, IgG1, IgG2a, IgG2b, IgG3, IgA and IgE were determined by ELISA. Statistical significance was determined with the unpaired t test for two-tailed data. *, significantly different between LMC and B-TRAF3-/- mice (p < 0.05); **, very significantly different between LMC and B-TRAF3-/- mice (p < 0.01). This figure has been modified from Xie et al.9. Please click here to view a larger version of this figure.
Figure 5: Representative results of TI and TD Ig isotype responses to TNP-polysaccharide and TNP-KLH immunization, respectively. Gender-matched, 8-12 weeks old LMC and M-TRAF3-/- mice (genetic background: C57BL/6) were immunized with 50 µg of the TI Ag TNP-polysaccharide (top panel, n = 9 for each genotype) or 100 µg of the TD Ag TNP-KLH mixed with alum (bottom panel, n = 12 for each genotype). Sera were collected on day 7 after immunization. Serum titers of anti-TNP IgM, IgG1, IgG2b, IgG3, IgA and IgE were analyzed by ELISA. Statistical significance was analyzed with the unpaired t test for two-tailed data. *, significantly different between LMC and M-TRAF3-/- mice (p < 0.05). This figure has been modified from Lalani et al.11. Please click here to view a larger version of this figure.
Figure 6: Representative results of TD primary and memory Ig responses to TNP-KLH immunization. Gender-matched, 8-10 weeks old LMC and T-TRAF3-/- mice (n = 10 for each genotype; genetic background: 129xC57BL/6) were immunized with 100 µg of the TD Ag TNP-KLH mixed with alum on day 0. Each mouse also received a booster immunization with the same Ag and adjuvant on day 21 after the first immunization. Serum samples were collected from mice on day -7, 7, 14 and 28, respectively. TNP-specific IgM and IgG1 levels in serum samples were measured by ELISA. Graphs depict the results of 10 pairs of LMC and T-TRAF3-/- mice (mean ± SD). Statistical significance was analyzed with the unpaired t test for two-tailed data. *, significantly different between LMC and T-TRAF3-/- mice (p < 0.05); **, very significantly different between LMC and T-TRAF3-/- mice (p < 0.01); ***, highly significantly different between LMC and T-TRAF3-/- mice (p < 0.001). This figure has been modified from Xie et al.10. Please click here to view a larger version of this figure.
Discussion
Here, we describe the protocol for the characterization of TD and TI Ig isotype responses in mice using ELISA. Successful implementation of this protocol requires the use of materials specified in Table 1, including ELISA assay plates, immunization Ags, mouse Ig isotype-specific antibodies and standards. Care should be taken to avoid using tissue culture treated plates for ELISA. Dilutions of the standards and serum samples should be done in separate untreated plates (round-bottom) and then added into the ELISA plates. Using this protocol, we have consistently obtained reliable results with high specificity and sensitivity.
Critical steps within this protocol include Ag immunization, retro-orbital bleeding, and Ig isotype-specific ELISA. For TD Ag immunization, TNP-KLH/alum mix should be thoroughly resuspended to ensure that correct amount of Ag/adjuvant is injected. If blood clots in the pipet or capillary tube during retro-orbital bleeding, immediately change to a new pipet or capillary tube. Buffers and reagents used in ELISA should be clear solutions and should not contain precipitates, which may give false positive results with abnormal OD405 values. In addition, bubbles should be avoided in the wells at all ELISA steps, which may also give false positive or false negative results with abnormal OD405 values. These occasional errors can be minimized by analyzing samples in duplicates. Washing steps in ELISA remove the unbound reagents and antibodies from the wells. Insufficient washing causes high background noise, but excessive washing may lead to a decrease in sensitivity by removing coated antigens or bound antibodies29. The numbers of wash times described in this ELISA protocol are optimized based on our experience.
It should be noted that ELISA has detection limits, which are usually defined by the linear range of the standard curves. In order to correctly quantify the serum concentration of each Ig isotype, the samples need to be appropriately diluted to fit within the linear range of the standard curves. We recommend testing serial dilutions of the samples to select the appropriate dilution factors for the calculation of Ig concentration as described in this protocol. However, if the results show that the dilution factors tested do not give results in the linear range of the standard curve, additional ELISA need to be performed using dilution factors adjusted according to the initial ELISA results. If all the dilutions tested are below the lower detection limit, original serum samples and smaller dilution factors need to be used. If OD405 values do not show proportional decrease with increasing dilution factor for all serial dilutions tested, this indicates that the capture Ab or coating Ag is saturated by all diluted samples and further higher dilution factors need to be used. Following this protocol, conclusive results will be obtained with at most 2 rounds of ELISA.
Factors that are known to influence antibody responses in mice include the strain (genetic background), gender, age, diet and animal facility environment30,31,32,33,34. For example, although many mouse strains produce IgG2a, certain strains such as C57BL/6 mice do not produce IgG2a but produce IgG2c instead35,36. Recent evidence also identifies commensal microbiota as a factor affecting antibody responses37,38,39,40. Taken these factors into consideration, we recommend the use of gender-matched, young adult littermates of different genotypes that share the same parents and cages for TD and TI Ag immunization experiments. In addition, mouse to mouse variation is frequently observed for mice of the same genotype (Figure 4-6), sufficient replicate numbers (typically, n > 8) of mice are needed for each genotype or group to obtain statistically meaningful results.
The Ig isotype-specific ELISA is useful in determining the titers of different Ig isotypes. However, this method alone is not sufficient to reveal the underlying causes of observed differences in Ig isotype titers, and therefore is often used in combination with a variety of complementary approaches. To differentiate whether the difference in Ig isotype titers is caused by different numbers of Ig-producing B cells or different efficiency of B cells at Ig production, flow cytometry41,42,43 and Enzyme-Linked ImmunoSpot (ELISPOT)43,44,45 can be used. To analyze B cell survival, proliferation and germinal center formation, alternative methods include flow cytometry, in vitro culture of splenic B cells, and immunohistochemical staining followed by microscopy9,26,41,46,47. To elucidate the changes in Ig isotype switching responses in B cells, in vitro culture of splenic B cells and quantitative RT-PCR of germline transcripts of Ig heavy chain gene segments are commonly used41,43,48,49. To investigate the cause of differences in affinity maturation, somatic hypermutation (SHM) of the Ig heavy chain gene is determined by sequencing of the VDJ region50,51,52. To understand differences in memory B cell responses, the frequency and number of different memory B cell subsets after Ag immunization can be analyzed by flow cytometry using recently identified markers of mouse memory B cells, including CD38, CD80, CD73, PD-L2, CD62L and CCR68,53,54,55. Together, these complementary methods used in combination with the current protocol will allow researchers to gain a comprehensive understanding of antibody responses in a given experimental setting.
Disclosures
The authors have no competing financial interests.
Acknowledgments
This study was supported by the National Institutes of Health grants R01 CA158402 (P. Xie) and R21 AI128264 (P. Xie), the Department of Defense grant W81XWH-13-1-0242 (P. Xie), a Pilot Award from the Cancer Institute of New Jersey through Grant Number P30CA072720 from the National Cancer Institute (P. Xie), a Busch Biomedical Grant (P. Xie), a Victor Stollar Fellowship (A. Lalani), and an Anne B. and James B. Leathem Fellowship (S. Zhu).
References
- Moise A, Nedelcu FD, Toader MA, Sora SM, Tica A, Ferastraoaru DE, Constantinescu I. Primary immunodeficiencies of the B lymphocyte. Journal of Medicine and Life. 2010;3:60–63. [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Haxhinasto SA, Stunz LL, Hostager BS. Antigen-specific B-lymphocyte activation. Critical Reviews in Immunology. 2003;23:149–197. doi: 10.1615/critrevimmunol.v23.i3.10. [DOI] [PubMed] [Google Scholar]
- Murphy K. Janeway's Immunobiology. 8th Edition. 2012;1 [Google Scholar]
- Vinuesa CG, Chang PP. Innate B cell helpers reveal novel types of antibody responses. Nature Immunology. 2013;14:119–126. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annual Review of Immunology. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Garcia de Vinuesa C, O'Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. European Journal of Immunology. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nature Reviews Immunology. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kraus ZJ, Stunz LL, Liu Y, Bishop GA. TNF Receptor-Associated Factor 3 Is Required for T Cell-Mediated Immunity and TCR/CD28 Signaling. The Journal of Immunology. 2011;186:143–155. doi: 10.4049/jimmunol.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani AI, Moore CR, Luo C, Kreider BZ, Liu Y, Morse HC, Xie P. Myeloid Cell TRAF3 Regulates Immune Responses and Inhibits Inflammation and Tumor Development in Mice. The Journal of Immunology. 2015;194:334–348. doi: 10.4049/jimmunol.1401548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Network. 2015;15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunological Reviews. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- Gan SD, Patel KR. Enzyme immunoassay and enzyme-linked immunosorbent assay. Journal of Investigative Dermatology. 2013;133:12. doi: 10.1038/jid.2013.287. [DOI] [PubMed] [Google Scholar]
- Shah K, Maghsoudlou P. Enzyme-linked immunosorbent assay (ELISA): the basics. British Journal of Hospital Medicine. 2016;77:98–101. doi: 10.12968/hmed.2016.77.7.C98. [DOI] [PubMed] [Google Scholar]
- Nencini F, Pratesi S, Petroni G, Matucci A, Maggi E, Vultaggio A. Assays and strategies for immunogenicity assessment of biological agents. Drug Development Research. 2014;75:4–6. doi: 10.1002/ddr.21184. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Haber E, Page LB, Richards FF. Radio immunoassay employing gel filtration. Analytical Biochemistry. 1965;12:163–172. doi: 10.1016/0003-2697(65)90155-7. [DOI] [PubMed] [Google Scholar]
- Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. 1960. Obesity Research. 1996;4:583–600. doi: 10.1002/j.1550-8528.1996.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA) Clinical Chemistry. 2005;51:2415–2418. doi: 10.1373/clinchem.2005.051532. [DOI] [PubMed] [Google Scholar]
- Wreghitt TG, Tedder RS, Nagington J, Ferns RB. Antibody assays for varicella-zoster virus: comparison of competitive enzyme-linked immunosorbent assay (ELISA), competitive radioimmunoassay (RIA), complement fixation, and indirect immunofluorescence assays. Journal of Medical Virology. 1984;13:361–370. doi: 10.1002/jmv.1890130407. [DOI] [PubMed] [Google Scholar]
- Mathew BC, Biju RS, Thapalia N. An overview of electrochemiluminescent (ECL) technology in laboratory investigations. Kathmandu University Medical Journal. 2005;3:91–93. [PubMed] [Google Scholar]
- Mikulskis A, Yeung D, Subramanyam M, Amaravadi L. Solution ELISA as a platform of choice for development of robust, drug tolerant immunogenicity assays in support of drug development. The Journal of Immunological Methods. 2011;365:38–49. doi: 10.1016/j.jim.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Wadhwa M, Bird C, Dilger P, Gaines-Das R, Thorpe R. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. The Journal of Immunological Methods. 2003;278:1–17. doi: 10.1016/s0022-1759(03)00206-0. [DOI] [PubMed] [Google Scholar]
- Wolforth JB. Methods of Blood Collection in the Mouse. Laboratory Animals. 2000;29:47–53. [Google Scholar]
- Stunz LL, Busch LK, Munroe ME, Sigmund CD, Tygrett LT, Waldschmidt TJ, Bishop GA. Expression of the Cytoplasmic Tail of LMP1 in Mice Induces Hyperactivation of B Lymphocytes and Disordered Lymphoid Architecture. Immunity. 2004;21:255–266. doi: 10.1016/j.immuni.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Xie P. TRAF molecules in cell signaling and in human diseases. Journal of Molecular Signaling. 2013;8:7. doi: 10.1186/1750-2187-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani AI, Zhu S, Gokhale S, Jin J, Xie P. TRAF molecules in inflammation and inflammatory diseases. Current Pharmacology Reports. 2018;4:64–90. doi: 10.1007/s40495-017-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J, Ward G. Interferences in immunoassay. The Clinical Biochemist Reviews. 2004;25:105–120. [PMC free article] [PubMed] [Google Scholar]
- Specter S, Friedman H. Age- and sex-related differences in antibody formation and blastogenic responsiveness of splenocytes from RIII mice developing virus-induced mammary adenocarcinoma. The Journal of the National Cancer Institute. 1981;67:1347–1351. [PubMed] [Google Scholar]
- Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski DA, Stavnezer J. Antibody class switching differs among SJL, C57BL/6 and 129 mice. International Immunology. 2007;19:545–556. doi: 10.1093/intimm/dxm020. [DOI] [PubMed] [Google Scholar]
- Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Veterinary Pathology. 2012;49:32–43. doi: 10.1177/0300985811429314. [DOI] [PubMed] [Google Scholar]
- Conour LA, Murray KA, Brown MJ. Preparation of animals for research--issues to consider for rodents and rabbits. Institute of Laboratory Animal Resources Journal. 2006;47:283–293. doi: 10.1093/ilar.47.4.283. [DOI] [PubMed] [Google Scholar]
- Vlkova M, Rohousova I, Hostomska J, Pohankova L, Zidkova L, Drahota J, Valenzuela JG, Volf P. Kinetics of antibody response in BALB/c and C57BL/6 mice bitten by Phlebotomus papatasi. PLOS Neglected Tropical Diseases. 2012;6:1719. doi: 10.1371/journal.pntd.0001719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. The Journal of Immunology. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Ruane D, Chorny A, Lee H, Faith J, Pandey G, Shan M, Simchoni N, Rahman A, Garg A, Weinstein EG, et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. The Journal of Experimental Medicine. 2016;213:53–73. doi: 10.1084/jem.20150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Puga I, Cerutti A. Innate signaling networks in mucosal IgA class switching. Advances in Immunology. 2010;107:31–69. doi: 10.1016/B978-0-12-381300-8.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praet JT, Donovan E, Vanassche I, Drennan MB, Windels F, Dendooven A, Allais L, Cuvelier CA, van de Loo F, Norris PS, et al. Commensal microbiota influence systemic autoimmune responses. The EMBO Journal. 2015;34:466–474. doi: 10.15252/embj.201489966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QN, Himes JE, Martinez DR, Permar SR. The Impact of the Gut Microbiota on Humoral Immunity to Pathogens and Vaccination in Early Infancy. PLOS Pathogens. 2016;12:1005997. doi: 10.1371/journal.ppat.1005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevan-Raj BP, Robert I, Heyer V, Page A, Wang JH, Cammas F, Alt FW, Losson R, Reina-San-Martin B. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. The Journal of Experimental Medicine. 2011;208:1649–1660. doi: 10.1084/jem.20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Getahun A, Chen X, Dollin Y, Cambier JC, Wang JH. Imbalanced PTEN and PI3K Signaling Impairs Class Switch Recombination. The Journal of Immunology. 2015;195:5461–5471. doi: 10.4049/jimmunol.1501375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boboila C, Yan C, Wesemann DR, Jankovic M, Wang JH, Manis J, Nussenzweig A, Nussenzweig M, Alt FW. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. The Journal of Experimental Medicine. 2010;207:417–427. doi: 10.1084/jem.20092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah HB, Koelsch KA. B-Cell ELISPOT: For the Identification of Antigen-Specific Antibody-Secreting Cells. Methods in Molecular Biology. 2015;1312:419–426. doi: 10.1007/978-1-4939-2694-7_42. [DOI] [PubMed] [Google Scholar]
- Bonsignori M, Moody MA. Simultaneous Detection of Antigen-Specific IgG- and IgM-Secreting Cells with a B Cell Fluorospot Assay. Cells. 2012;1:15–26. doi: 10.3390/cells1010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Goodlad JR, Macartney JC. Germinal-center cell proliferation in response to T-independent antigens: a stathmokinetic, morphometric and immunohistochemical study in vivo. European Journal of Immunology. 1995;25:1918–1926. doi: 10.1002/eji.1830250719. [DOI] [PubMed] [Google Scholar]
- Xie P, Poovassery J, Stunz LL, Smith SM, Schultz ML, Carlin LE, Bishop GA. Enhanced Toll-like receptor (TLR) responses of TNFR-associated factor 3 (TRAF3)-deficient B lymphocytes. Journal of Leukocyte Biology. 2011;90:1149–1157. doi: 10.1189/jlb.0111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku H, Horikawa K, Obata Y, Kato I, Okamoto H, Sakaguchi N, Gerondakis S, Takatsu K. NF-kappaB is required for CD38-mediated induction of C(gamma)1 germline transcripts in murine B lymphocytes. International Immunology. 2002;14:1055–1064. doi: 10.1093/intimm/dxf072. [DOI] [PubMed] [Google Scholar]
- Dudley DD, Chaudhuri J, Bassing CH, Alt FW. Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences. Advances in Immunology. 2005;86:43–112. doi: 10.1016/S0065-2776(04)86002-4. [DOI] [PubMed] [Google Scholar]
- Lange H, Hecht O, Zemlin M, Trad A, Tanasa RI, Schroeder HW, Lemke H. Immunoglobulin class switching appears to be regulated by B-cell antigen receptor-specific T-cell action. European Journal of Immunology. 2012;42:1016–1029. doi: 10.1002/eji.201141857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CR, Liu Y, Shao CS, Covey LR, Morse HC, Xie P. Specific deletion of TRAF3 in B lymphocytes leads to B lymphoma development in mice. Leukemia. 2012;26:1122–1127. doi: 10.1038/leu.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann B, Grimsholm O, Thorarinsdottir K, Ren W, Jirholt P, Gjertsson I, Martensson IL. Memory B cells in mouse models. Scandinavian Journal of Immunology. 2013;78:149–156. doi: 10.1111/sji.12073. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nature Immunology. 2015;16:296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgueta R, Marks E, Nowak E, Menezes S, Benson M, Raman VS, Ortiz C, O'Connell S, Hess H, Lord GM, et al. CCR6-dependent positioning of memory B cells is essential for their ability to mount a recall response to antigen. The Journal of Immunology. 2015;194:505–513. doi: 10.4049/jimmunol.1401553. [DOI] [PMC free article] [PubMed] [Google Scholar]


