Abstract
Pembrolizumab monotherapy has been approved for the first- and second-line treatment of patients with PD-L1-expressing advanced non-small cell lung cancer (NSCLC). Testing for PD-L1 expression with the PD-L1 immunohistochemistry (IHC) 22C3 companion diagnostic assay, which gives a tumor proportion score (TPS), has been validated on tumor tissue. We developed an optimized laboratory-developed test (LDT) that uses the 22C3 antibody (Ab) concentrate on a widely available IHC autostainer for biopsy and cytology specimens. The PD-L1 TPS was evaluated with 120 paired whole-tumor tissue sections and biopsy samples and with 70 paired biopsy and cytology samples (bronchial washes, n = 40; pleural effusions, n = 30). The 22C3 Ab concentrate-based LDT showed a high concordance rate between biopsy (~100%) and cytology (~95%) specimens when compared to PD-L1 IHC expression determined using the PD-L1 IHC 22C3 companion assay at both TPS cut points (≥1%, ≥50%). The optimized LDT presented here, using the 22C3 Ab concentrate to determine the PD-L1 expression in both tumor tissue and in cytology specimens, will expand the ability of laboratories worldwide to assess the eligibility of patients with NSCLC for treatment with pembrolizumab monotherapy in a reliable and reproducible manner.
Keywords: Cancer Research, Issue 139, PD-L1, LDT, immunohistochemistry, biopsy, cytology, non-small cell lung cancer
Introduction
Recent clinical trials have demonstrated the efficacy of pembrolizumab, a humanized monoclonal IgG4 kappa isotype antibody that blocks the interaction between programmed cell death 1 (PD-1) and its ligands, PD-L1 and PD-L2, in the treatment of patients with advanced NSCLC1,2,3,4.
Currently, pembrolizumab is approved for treatment of PD-L1-expressing NSCLC in both treatment-naive patients with a PD-L1 expression TPS of ≥50% and no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations3 and for previously treated patients with a PD-L1 TPS of ≥1%1.
PD-L1 protein expression detected by IHC has been widely used as a predictive biomarker assay for anti-PD-1/PD-L1 therapies. In pembrolizumab clinical trials, the PD-L1 TPS obtained with formalin-fixed paraffin-embedded (FFPE) tissue samples was determined using the PD-L1 IHC 22C3 companion assay5. This assay has been approved by the US Food and Drug Administration (FDA) and has been CE-marked in Europe for the determination of the tumor PD-L1 TPS5.
Additional global options across institutions for reliable and high-quality evaluations of the PD-L1 TPS with LDTs which use the 22C3 antibody concentrate are essential to support clinical decisions made regarding patient eligibility for pembrolizumab treatment. A large number of pathology laboratories do not have access to the companion diagnostic PD-L1 IHC 22C3 assay. Therefore, the development of reliable and consistent LDTs compatible with additional, widely available IHC autostainer platforms is essential.
Moreover, there is a need to establish LDTs using cytology samples that are the only specimen type frequently available from NSCLC patients. The PD-L1 IHC 22C3 companion assay is validated for resections, core needle biopsies, and bronchoscopies only if the bronchoscopy yields 100 tumor cells. Although the above sample types are frequently obtained, cytology samples are more easily collected and are the most commonly available sample type in some institutions6,7. However, there is currently no validated diagnostic assay available for the evaluation of the PD-L1 expression in cytology samples; reliable LDTs compatible with cytology samples would further facilitate high-quality PD-L1 testing.
Furthermore, when establishing the clinical validation of an LDT, the IHC should be performed in a similar way to the corresponding clinically validated commercial test8. For instance, several critical steps should be verified to obtain the same signal in serial sections such as antibody titration, pretreatment delays, incubation time, and amplification systems9.
We recently developed an optimized LDT that uses the 22C3 antibody concentrate to evaluate the PD-L1 expression on tumor biopsies and cytology samples10,11. We found a high concordance with the LDT versus the "gold standard" PD-L1 IHC 22C3 assay10,11. This clinically validated protocol will support reliable, high-quality PD-L1 testing across regions globally.
Protocol
All procedures have been approved by the local ethics committee (Human Research Ethics Committee, Centre Hospitalier Universitaire de Nice/Tumorothèque BB-0033-00025).
NOTE: This protocol is specifically adjusted for the use of the 22C3 antibody concentrate on a commercially available automated IHC stainer (referred to as autostainer here, see the Table of Materials) for tumor biopsies and cytology samples.
1. Preparation of Tumor Tissue Samples
Fix tumor tissue in 10% neutral buffered formalin (NBF) in a cassette. NOTE: Tumor tissue from proximal lung tumors is generally obtained by bronchoscopy, performed under moderate sedation by a pulmonologist, or lung specialist. For peripheral lesions and diffuse lung disease, a transbronchial or needle biopsy is indicated. These procedures are usually done in a surgery room or intensive care unit.
Embed the cassette in paraffin. NOTE: Fixation can be achieved by perfusion or immersion immediately following dissection, and typically requires 8–10 h. The fixative volume should be 15–20x higher than the specimen volume. It is not recommended to fix biopsy tissue for more than 10 h, because overfixation can cause masking of the antigen. The fixation speed is about 1 mm/h at room temperature.
Infiltrate the fixed tissue sample with wax in the tissue processor (see Table of Materials). NOTE: Dehydration is performed in three alcohol baths with increasing concentrations 70%, 85%, and 90%. Water is finally removed by three final absolute alcohol baths. The clearing is performed in three toluene baths, and the wax infiltration in hot wax baths (44–60 °C).
Embed the tissue inside a mold filled with molten paraffin (see Table of Materials) and wait for solidification.
Section the paraffin-embedded tissue at a thickness of 3 μm on a microtome.
Transfer the paraffin ribbon to a positively charged microscope glass slide (see Table of Materials).
Dry the slide for 1 h at 37 °C. Note: Store the tissue sections at 2–8 °C (preferred) or at room temperature up to 25 °C to preserve antigenicity, and stain within 15 d of sectioning.
2. Preparation of Cytology Samples
- Collect bronchial washings in a preservative solution (see Table of Materials). NOTE: For this, standard bronchoscopy technique is used.
- Lavage the distribution of the bronchus to be sampled. Collect the wash in a clean container. Label the container with the patient’s first and last name, date of birth, and specimen source.
Transfer the bronchial washings to a 50 mL conical tube and add 2 g of DL-Dithiothreitol powder (see Table of Materials), vortex the tube for 30 min, and then centrifuge it at 250 x g for 5 min at room temperature.
Remove the supernatant and add 10 mL of a mucolytic solution (see Table of Material).
Shake the sample for 20 min at medium speed (level 9) and centrifuge it at 250 x g for 5 min at room temperature.
Remove the supernatant and deposit the cell pellet in collection tubes containing 10% NBF.
Add 4 drops of a cell block preparation reagent (see Table of Materials) to the cell pellet.
Transfer the cell pellet to a cassette, fix it in 10% NBF, and paraffin-embed it for cell block preparation and sectioning (see steps 1.1–1.7).
3. PD-L1 Staining Assay
Switch on the autostainer and the computer.
Double-click on the autostainer icon and choose the user.
Click on 'Create label' and then on 'Protocol'.
Double-click on the 22C3 protocol. NOTE: The protocol is first installed on the autostainer’s computer by clicking on 'Create Protocol'. The full protocol is provided as Supplementary File 1.
Write the patient’s ID on the label and click on 'Print'.
Stick the label on the slide.
Open the slide drawer by pressing on the button of the drawer that has been chosen.
Place the labeled slide on the thermal pad with the label facing up- and inward.
Close the slide drawer.
Remove the caps from the dispensers and load the reagents on the reagents’ racks.
Open the hood of the stainer and place the racks on the reagents’ carrousel making sure that they fit and hold in position.
Close the hood.
On the software, click on the instrument that will be used and click on 'Running'.
At the end of the IHC procedure, rinse the slide for several seconds with tap water and one drop of a cleaning solution.
Rehydrate the slide with one bath of ethanol 100% and a second bath of ethanol 95% for several seconds.
Place the slide into the coverslipping machine for an automated loading of the coverslip.
4. Interpretation of the PD-L1 Staining
NOTE: A qualified pathologist should perform the interpretation of the PD-L1 IHC test.
Assess the quality of the PD-L1 staining by analyzing the positive and negative controls before the examination of the patient’s specimen. NOTE: The results obtained on the patient’s specimen are considered invalid if the staining of the controls is not acceptable.
Confirm the presence of a minimum of 100 viable tumor cells under a microscope. NOTE: Report when the patient’s specimen has less than 100 tumor cells.
At a low magnification of 4X, evaluate all well-preserved positive and negative tumor areas. NOTE: At a low magnification, partial membrane staining or membrane staining of weak intensity (1+) may be difficult to recognize.
Score partial or complete cell membrane staining. NOTE: The cytoplasmic staining has to be excluded from interpretation.
Calculate the TPS by assessing the proportion of PD-L1 positive tumor cells relative to all tumor cells present in the well-preserved tumor areas. NOTE: Only viable tumor cells should be examined. All other cellular elements, such as immune cells, necrotic cells, normal cells, and artifacts, have to be excluded from the examination.
Representative Results
Using the procedure presented here, and as described in detail in this group's recent publications10,11, the optimized LDT was clinically validated with 120 archival FFPE NSCLC biopsy samples from patients who underwent surgical resection or a biopsy at the Pasteur University Hospital, Nice, between March 2007 and March 2016. Moreover, for the evaluation of PD-L1 expression of cytology samples, TPS was evaluated in 70 paired tissue biopsy samples and cell blocks that were prepared from bronchial washes (n = 40) or pleural effusions (n = 30) (collected at the Pasteur University Hospital, Nice, between July 2014 and November 2016). All the slides were freshly cut and stained within 24 hours.
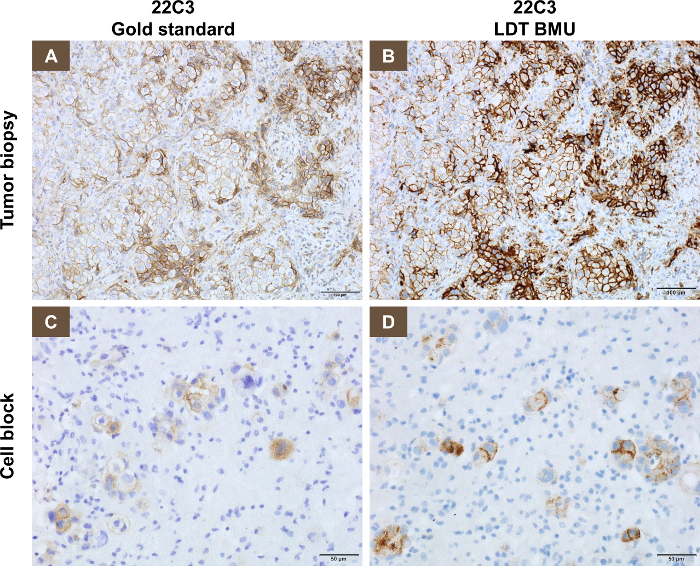
A representative staining pattern with biopsy specimens using the 22C3 antibody concentrate (the LDT), compared with the PD-L1 IHC 22C3 companion assay (gold standard) is shown in Figure 1A and 1B. Using a TPS of ≥1%, 54/120 cases (45%) were PD-L1 positive, while using a TPS of ≥50%, 29/120 cases (24%) were considered positive for PD-L1.
The intraclass correlation coefficient (ICC) used to measure the correlation of the TPS score as a continuous variable was 99% between the LDT and the gold standard. Using both the TPS cut points of ≥1% and ≥50%, the κ scores for interpathologist agreement were equal to 1 within the LDT platform.
A representative staining pattern with cytology specimens using the 22C3 antibody concentrate on the LDT compared with the PD-L1 IHC 22C3 kit (gold standard) is shown in Figure 1C and 1D. The concordance rate of 70 pairs of biopsy and cytology samples with the LDT using either one of the TPS cut points of ≥1% and ≥50% was greater than 95%, and the ICC using the TPS score as a continuous variable was between 0.88 and 0.90. This finding was consistent across each type of cytology sample (pleural effusion vs. bronchial wash) or tumor histology (adenocarcinoma vs. squamous cell carcinoma) with an ICC between 0.82 and 0.96. When comparing the PD-L1 TPS of 37 (out of 70) pairs of cytology samples with the LDT to the biopsy samples with the gold standard, the concordance rate using either the ≥1% TPS or the ≥50% TPS cut point was greater than 97% and the ICC was between 0.93 and 0.95.
Figure 1: Representative staining pattern on biopsy and cytology specimens using the 22C3 antibody concentrate (LDT), compared with the PD-L1 IHC 22C3 kit (gold standard). (A) This panel shows the PD-L1 IHC 22C3 assay used on a tumor biopsy. (B) This panel shows the optimized LDT using the 22C3 antibody concentrate on serial sections from the same tumor biopsy as analyzed in panel A. (C) This panel shows the PD-L1 IHC 22C3 assay in a cell block. (D) This panel shows the optimized LDT using the 22C3 antibody concentrate on serial sections from the same cell block as analyzed in panel C. Please click here to view a larger version of this figure.
Discussion
We have validated an optimized LDT using the 22C3 PD-L1 antibody concentrate, by comparing it with the corresponding clinically validated commercial test10,11. The 22C3 concentrated antibody-based LDT showed a high concordance rate between biopsy (~100%) and cytology (~95%) specimens when compared to the PD-L1 IHC expression determined using the PD-L1 IHC 22C3 assay at both ≥1% TPS and the ≥50% TPS cut points. As recently recommended by the International Association for the Study of Lung Cancer, the PD-L1 positive areas should be the same for approximately 10 PD-L1 negative samples, 10 PD-L1 positive samples, and 20 samples covering the linear dynamic range of the clinically validated PD-L1 IHC test8. We performed a validation study on 120 biopsies and 70 cytology samples. Overall, the concordance was high, independently of the TPS cutoff for positivity, the type of specimens, or tumor histology. The findings presented here are in agreement with those of other previous studies showing a high concordance rate for both tissue and cytology specimens12,13,14,15.
In this study, all the specimens were fixed in 10% NBF. We did not evaluate the impact of other fixatives, while the effect on PD-L1 staining of other non-formalin fixatives such as alcohol-based fixatives is currently unexplored. The presence of immune cells expressing PD-L1, particularly macrophages, warrants a careful interpretation of the cytology specimens. These samples must be evaluated with reference to the serial hematoxylin and eosin slide, and, in some difficult cases, complementary stains to assess immune cells may be performed to exclude any misinterpretation of the PD-L1 expression in tumor cells only.
This study holds a number of limitations, including a retrospective analysis at a single institution, and the fact that no patient was treated with pembrolizumab to evaluate the clinical outcome. In addition, a minor limitation of the LDT presented here concerns the granular staining pattern that was observed occasionally when using amplification systems, which may make their interpretation more difficult. Moreover, the staining pattern of immune cells is somewhat more intense than that observed with the gold standard. The training of pathologists is necessary to obtain a correct interpretation of PD-L1 staining with IHC.16
In the clinical setting, the robustness of LDTs needs to be maintained over time by seeking certification and accreditation, by following standard operating procedures, and by regularly participating in external quality assessment schemes8. The guarantee of the clinical predictive performance can be ensured only when these prerequisites are accomplished8.
The protocol presented here addresses the critical need for PD-L1 LDTs on both biopsy and cytology samples when analyzed on a widely available autostainer across geographic regions that may not be equipped with the gold standard assay. The LDT presented here, which was optimized using the 22C3 antibody concentrate, can be used to evaluate the PD-L1 expression on both biopsy and cytology samples from NSCLC patients. This will significantly expand the number of laboratories that can offer high-quality PD-L1 testing to identify patients with NSCLC who are eligible for treatment with pembrolizumab monotherapy, in a reliable and reproducible manner.
A potential future direction is to assess the feasibility of using other cytology specimen types (e.g., specimens from fine-needle biopsies, bronchoscopy-guided FNA, endobronchial ultrasound-guided FNA, and bronchial brushes) with 22C3 antibody concentrate-based LDTs.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was sponsored by Merck & Co., Inc., Kenilworth, NJ, USA. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Herbst RS, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- Reck M, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England Journal of Medicine. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- Langer CJ, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner R, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2017;35(34):3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- Folch E, Costa DB, Wright J, VanderLaan PA. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Translational Lung Cancer Research. 2015;4(4):392–403. doi: 10.3978/j.issn.2218-6751.2015.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, et al. Improving Adequacy of Small Biopsy and Fine-Needle Aspiration Specimens for Molecular Testing by Next-Generation Sequencing in Patients With Lung Cancer: A Quality Improvement Study at Dartmouth-Hitchcock Medical Center. Archives of Pathology & Laboratory Medicine. 2017;141(3):402–409. doi: 10.5858/arpa.2016-0096-OA. [DOI] [PubMed] [Google Scholar]
- Tsao MS, et al. IASLC ATLAS of PD-L1 Immunohistochemistry Testing in Lung Cancer. International Association for the Study of Lung Cancer (IASLC) Press; 2017. [Google Scholar]
- Thunnissen E, de Langen AJ, Smit EF. PD-L1 IHC in NSCLC with a global and methodological perspective. Lung Cancer. 2017. pp. 102–105. [DOI] [PubMed]
- Ilie M, et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One. 2017;12(8):e0183023. doi: 10.1371/journal.pone.0183023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie M, et al. Use of the 22C3 anti-programmed death ligand 1 antibody to determine programmed death ligand 1 expression in cytology samples obtained from non-small cell lung cancer patients. Cancer Cytopathology. 2018;126(4):264–274. doi: 10.1002/cncy.21977. [DOI] [PubMed] [Google Scholar]
- Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Applied Immunohistochemistry & Molecular Morphology. 2017;25(7):453–459. doi: 10.1097/PAI.0000000000000540. [DOI] [PubMed] [Google Scholar]
- Russell-Goldman E, Kravets S, Dahlberg SE, Sholl LM, Vivero M. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathology. 2018;126(4):253–263. doi: 10.1002/cncy.21973. [DOI] [PubMed] [Google Scholar]
- Heymann JJ, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathology. 2017;125(12):896–907. doi: 10.1002/cncy.21937. [DOI] [PubMed] [Google Scholar]
- Stoy SP, Rosen L, Mueller J, Murgu S. Programmed death-ligand 1 testing of lung cancer cytology specimens obtained with bronchoscopy. Cancer Cytopathology. 2018;126(2):122–128. doi: 10.1002/cncy.21941. [DOI] [PubMed] [Google Scholar]
- Ilie M, Hofman P. Reproducibility of PD-L1 assessment in non-small cell lung cancer-know your limits but never stop trying to exceed them. Translational Lung Cancer Research. 2017;6(Suppl 1):S51–S54. doi: 10.21037/tlcr.2017.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


