Fig. 5. MTX and CPT1 compete for binding to R243 on the KCNQ1 S4-S5 linker.
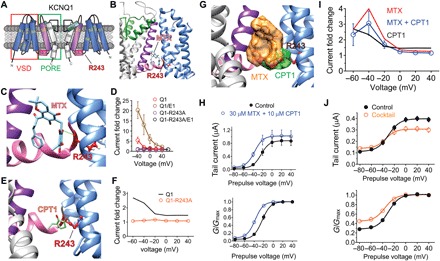
All error bars indicate SEM. (A) KCNQ1 topology (two of four subunits shown) indicating the position of R243 on the S4-S5 linker. (B) SwissDock result showing predicted binding of MTX to KCNQ1 via hydrogen bonding (green line) to R243. (C) Close-up of docking shown in (B). (D) Mean current augmentation by MTX versus voltage of wild-type and KCNQ1-R243A and KCNQ1-KCNE1 channels (n = 5 to 8). (E) SwissDock result showing predicted binding of CPT1 to KCNQ1-R243. (F) Mean current augmentation by CPT1 versus voltage of wild-type (from Fig. 2) and KCNQ1-R243A channels (n = 10). (G) SwissDock result showing predicted binding pose overlap in KCNQ1 of MTX and CPT1. (H) Mean effects of 30 μM MTX + 10 μM CPT1 on KCNQ1 raw tail currents (upper) and G/Gmax (lower) at −30 mV after prepulses as indicated (n = 5). (I) Mean current augmentation versus voltage of KCNQ1 by 30 μM MTX + 10 μM CPT1 (blue) [from (H)] compared to effects for MTX alone (red) or CPT1 alone (orange) (from Fig. 2) (n = 4 to 7). (J) Mean effects of M. oppositifolius leaf extract cocktail on KCNQ1 raw tail currents (upper) and G/Gmax (lower) at −30 mV after prepulses as indicated (n = 6).
