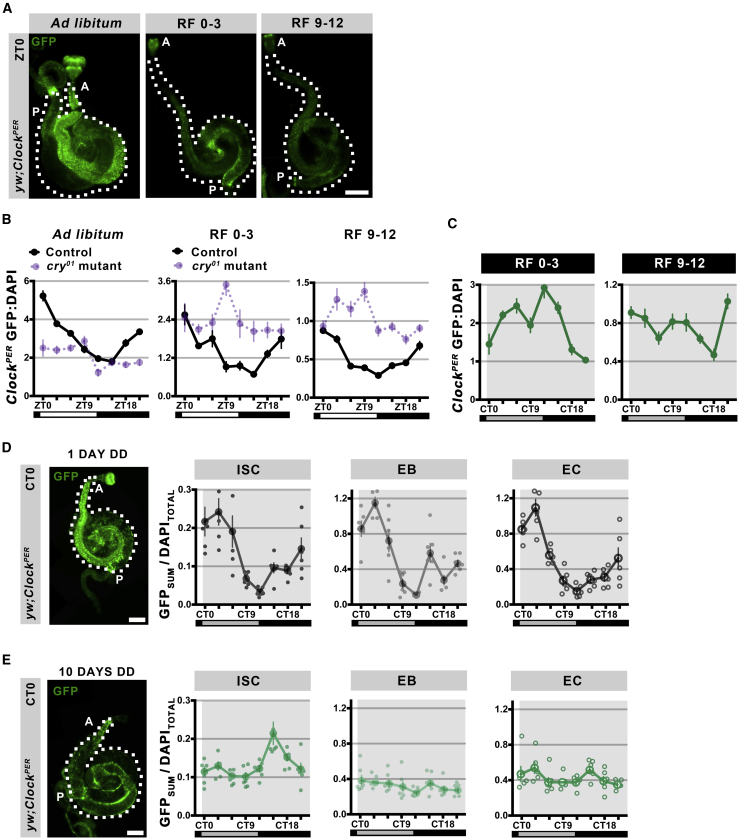
Figure 3.
Feeding Time Can Entrain the Drosophila Intestinal Clock
(A) Representative images of ClockPER intestines from flies fed ad libitum or restricted to ZT0-3 or ZT9-12. GFP+ signal is lower under restricted feeding. A, the anterior region; P, posterior. Scale bar represents 250 μm.
(B) Graphs of ClockPER GFP:DAPI signal in the whole intestine under LD photoperiod under ad libitum or restricted feeding. Control data (left graph) are the same as used in Figure 1F. Ad libitum-fed CRY mutants (cry01) show higher GFP levels during the daytime (ZT0-9) than night (ZT12-21), and are significantly different than controls (two-way ANOVA F = 13.15, p < 0.0001). Under restricted feeding, control intestines follow similar rhythms (albeit with different amplitude), with the same peaks and troughs irrespective of feeding regimen, suggesting that photoperiod is the key entrainment factor in the intestine. CRY mutants show distinct rhythms in these different regimens. cry01 are significant in RF0-3 (one-way ANOVA F = 2.417, p = 0.0270) and RF9-12 (one-way ANOVA F = 6.083, p < 0.0001); control versus cry01 is significant: RF0-3 (two-way ANOVA F = 5.092, p < 0.0001), RF9-12 (two-way ANOVA F = 9.63, p < 0.0001). Data presented as mean of n ≥ 10 intestines, error bars show ±SEM.
(C) Graphs of ClockPER GFP:DAPI signal in wild-type flies with 5 days of restricted feeding at CT0-3 versus CT9-12 (DD conditions). Alterations in clock reporter activity suggest the timing of feeding affects clock function in these otherwise free-running conditions. Data presented as mean of n ≥ 10 intestines, error bars show ±SEM. One-way ANOVA: control 0–3 (F = 12.48, p < 0.0001); control 9–12 (F = 2.161, p = 0.0467).
(D) Representative image of a ClockPER GFP+ Drosophila intestine at CT0, at 1 day DD. DAPI counterstains nuclei. A, the anterior region; P, posterior. Scale bar represents 250 μm. Graphs show quantification of cell-specific rhythms in the posterior (R5) region with EC, EB, and ISC signals analyzed separately. One day after LD photoperiod, all clock active intestinal cells exhibit synchronous circadian rhythms. GFPSUM is fluorescence from n = 5 cells of each cell type (ISC, EB, or EC) normalized to the DAPI coming from all cells quantified (n = 15 total). Data are the mean of n = 6 intestines, error bars show ±SEM. One-way ANOVA: ISC (F = 8.485, p < 0.0001), EB (F = 21.36, p < 0.0001), EC (F = 26.37, p < 0.0001).
(E) The same analysis carried out 10 days after DD. Following a long period of free-running conditions, intestinal cells are no longer synchronous and the average rhythm for each cell type is altered. One-way ANOVA: ISC (F = 6.283, p < 0.0001), EB (F = 1.332, p = 0.2608), EC (F = 2.083, p = 0.0679).