Table 1.
Chemical structure of CCR systems and evaluation of the reaction kinetics of the CCR system. All reactions were conducted in PBS containing 10% DMSO.
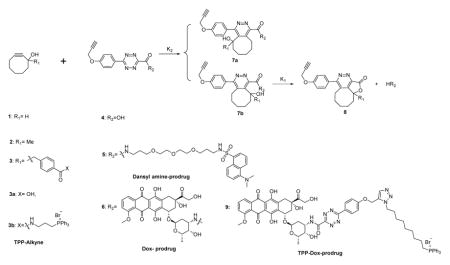
| |||||
|---|---|---|---|---|---|
| Tetrazine | Alkyne | k2 at r.t (M−1s−1) | k2 at 37 °C (M−1s−1) | k1 at r.t (h−1) | Dox Peak % within 48 h, at r.t (%) |
| 5 | 1 | 0.25± 0.06 | 1.9± 0.4 | 0.029± 0.006 | 60± 6 (48 h) |
| 2 | 0.0075± 0.0009 | 0.021± 0.005 | * | 90± 5 (20 h) | |
| 3b | 0.042± 0.012 | 0.14± 0.04 | 0.16±0.04 | 90 ±3 (16 h) | |
| 6 | 1 | 0.36± 0.07 | 2.1 ±0.4 | Δ | 20±5 (48 h) |
| 2 | 0.0078±0.0009 | 0.025±0.006 | * | 85±5 (21 h) | |
| 3b | 0.065±0.013 | 0.19±0.04 | 0.20±0.04 | 80±5 (18 h) | |
| 3a | 0.061±0.013 | 0.17±0.05 | 0.21±0.04 | 80±5 (19 h) | |
| 9 | 3b | 0.091±0.013 | 0.25±0.05 | 0.22±0.05 | 78±6 (18 h) |
not detectable because of the slow second order reaction and fast lactonization reaction. No intermediates were observed.
not detectable because of Dox decomposition in PBS. (n = 3, p= 0.95).
