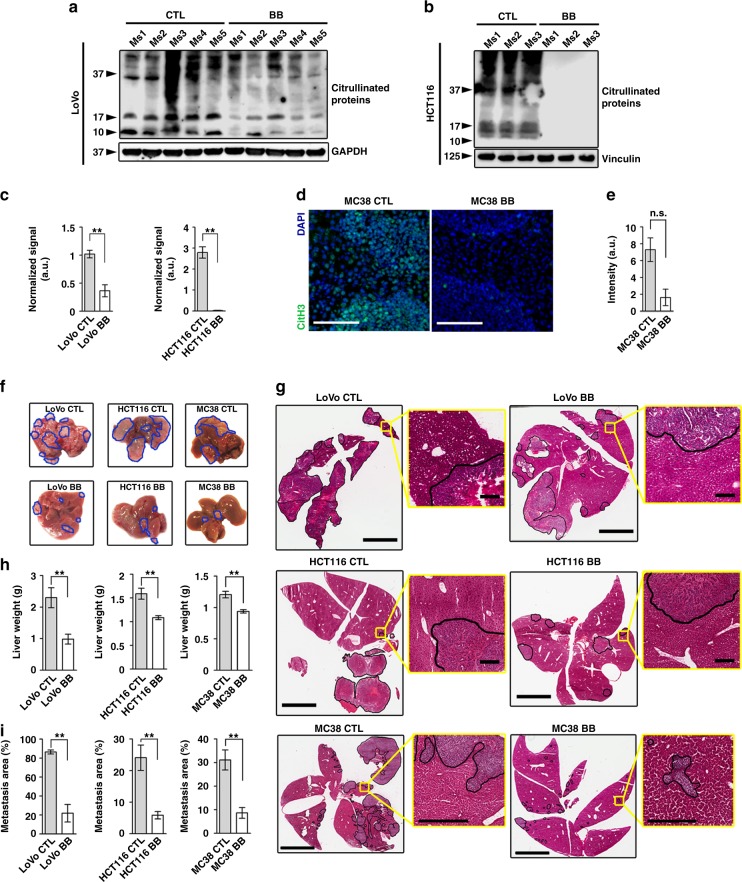
Fig. 5.
Pharmacological inhibition of PADs diminishes experimental hepatic metastases in vivo. a, b Immunoblotting for citrullinated proteins performed on tissue lysates extracted from experimental LoVo and HCT116 hepatic metastases from mice treated with vehicle or BB-Cl-amidine. Each lane indicates a biological replicate. GAPDH and Vinculin were used as a loading control. c Densitometric analysis for (a) and (b). d Representative images of immunostaining for citrullinated Histone H3 in experimental MC38 hepatic metastases from mice treated with vehicle (n = 5 biological replicates), or BB-Cl-amidine (n = 6 biological replicates). n = 3 sections per group, three images were analyzed per section. Scale bar = 100 μm. e Staining intensity quantification for (d). f Representative images of livers with experimental LoVo, HCT116 and MC38 hepatic metastases from mice treated with vehicle (n = 7, 6, and 5 biological replicates, respectively) or BB-Cl-amidine (n = 8, 6, and 6 biological replicates, respectively). Metastatic nodules are outlined in blue. Representative of two experiments. g Representative H&E-stained and scanned slides of dissected livers from the experiment in (f). Metastatic regions are outlined in black. Scale bar = 4 mm (scale bar of inserts = 200 μm). h Weight of excised metastasis-bearing livers from the experiment in (f). i Metastatic area based on the assessment of H&E-stained sections of livers from the experiment in (f). At least three different sections per mouse were analyzed. Throughout, error bars indicate s.e.m. center values indicate mean (**P < 0.01, n.s. = non-significant, Mann–Whitney U test). CTL control, BB BB-Cl-amidine