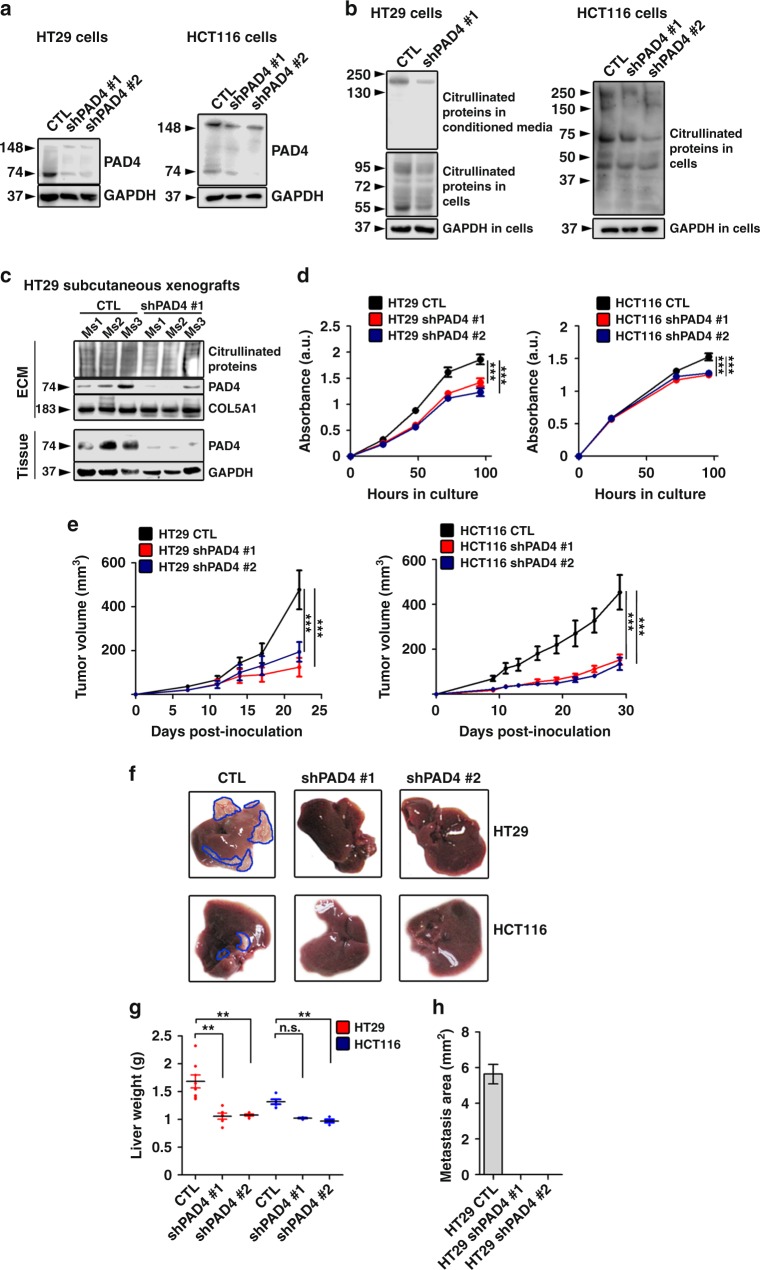
Fig. 7.
Genetic downregulation of PAD4 diminishes proliferation and subcutaneous tumor growth of CRC cells, and reduces experimental metastasis in vivo. a Immunoblotting for PAD4 in HT29 and HCT116 cells transfected with lentivirus harboring scrambled shRNA (CTL) or shPAD4 (shPAD4 #1 and #2). GAPDH was used as a loading control. b Immunoblotting for citrullinated proteins in conditioned media and cell lysates from control and PAD4-deficient HT29 and HCT116 cells. GAPDH was used as a loading control. c Immunoblotting analysis for PAD4 and citrullinated proteins in tissue lysates and isolated ECM from control (CTL) and PAD4 knockdown (shPAD#1) subcutaneous xenografts. GAPDH and COL5A1 were used as loading controls for tissue lysates and isolated ECM, respectively. d WST-1 proliferation and viability assay performed in cultured wild-type and PAD4-deficient HT29 and HCT116 cells (n = 5 technical replicates per group). e Tumor growth curves of mice injected subcutaneously with control and PAD4-knockdown HT29 and HCT116 cells (n = 7, 4, and 5 biological replicates for HT29 and 9, 5, and 5, for HCT116, respectively). Repeated twice. f Representative images of livers from mice injected intrasplenically with control (n = 8 biological replicates) or PAD4-deficient HT29 and HCT116 cells (n = 6 and 6 biological replicates for shPAD4 #1 and #2) 35 days postinjection. Metastatic nodules are outlined in blue. g Liver weights from the experiment in (f). h Measurement of the area of metastatic deposits in HT29 livers from the experiment in (f). For g, error bars indicate s.e.m. center values indicate mean (**P < 0.01, Kruskal–Wallis test with Dunn’s multiple comparison post-test). For d and e, error bars indicate s.e.m. center values indicate mean (***P < 0.001, two-way ANOVA). Ms mouse, CTL control, shPAD4 PAD4 knockdown, ECM extracellular matrix