Fig. 5.
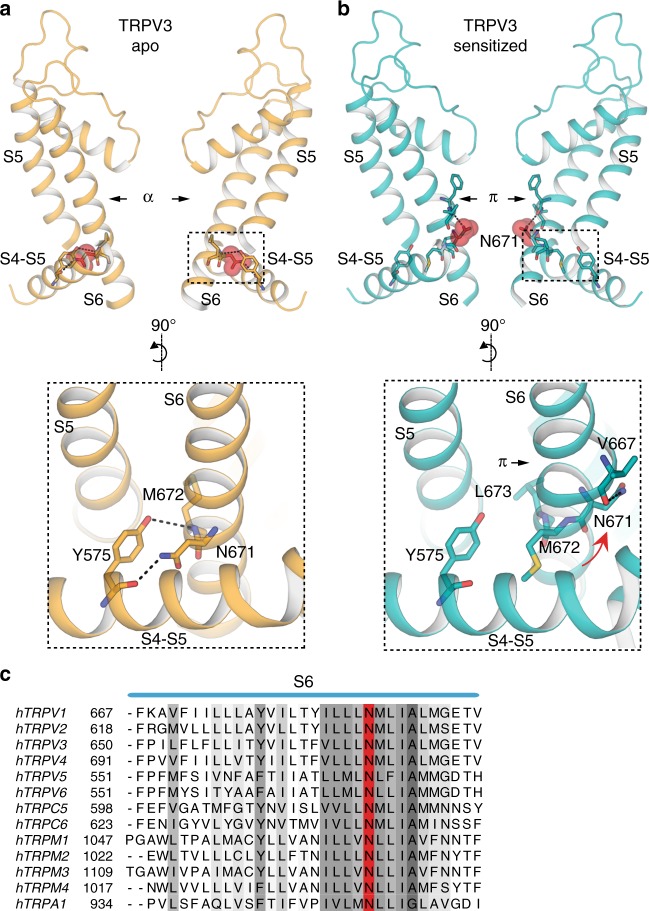
Formation of the π-helix in S6 changes the S4−S5 linker/pore domain interface. a The register of the α-helical S6 in the apo TRPV3 enables an interaction network between the Y575 in the S4−S5 linker and N671 and M672 in S6. Residue N671 is shown in sphere representation in top panel. b Formation of a π-helix in the sensitized state, changes the register of the helix, so that N671 turns away from the S4−S5 linker and into the pore, where it interacts with the backbone of V667 residue in the π-helix. Residue N671 is shown in sphere representation in top panel. c Sequence alignment of TRP channel S6 helices. The conserved N residue (N671 in TRPV3) is marked in red