Figure 2.
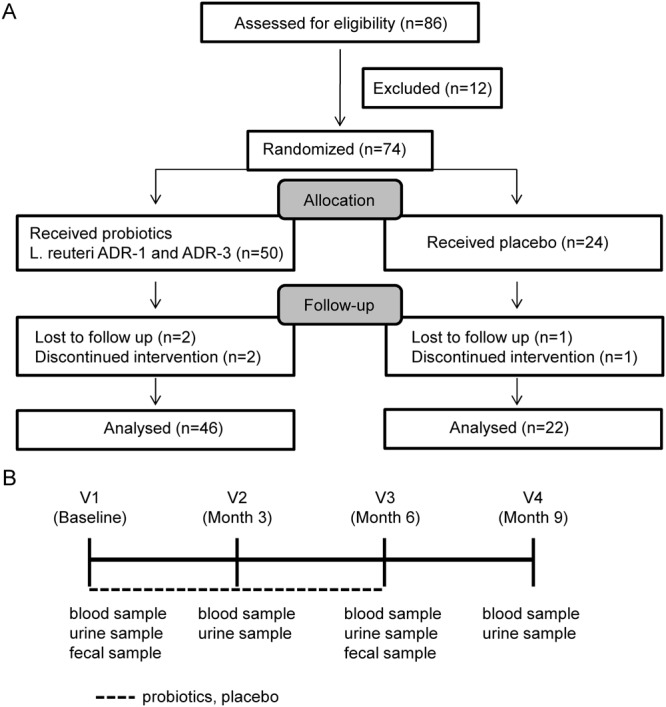
Recruitment of participants and the study design of a double blinded, randomized, and placebo controlled trial. (A) A total of 86 T2DM patients came for assessment of eligibility and excluded for 12 people. A total of 74 eligible participants was randomized divided into live L. reuteri ADR-1, heat-killed L. reuteri ADR-3, or placebo group. After exclusion for participates with loss of follow up or discontinued intervention, there was 46 or 22 analyzed participates for probiotics consumption or placebo group, respectively. (B) The probiotics consumption was performed a total of 6 months and wash out the probiotics for further 3month. All participates were requested to collect fasting blood and urine samples at 4 visits (V1: starting point, V2: 3 months after consumption, V3: 6 months after consumption, and V4: 3 months after stopping consumption) as indicated. The fecal samples were collected at V1 and V3.
