Figure EV3. The H5‐1/2 loop is flipped toward the C‐terminus of the ARM domain in Arp2/3‐bound SPIN90.
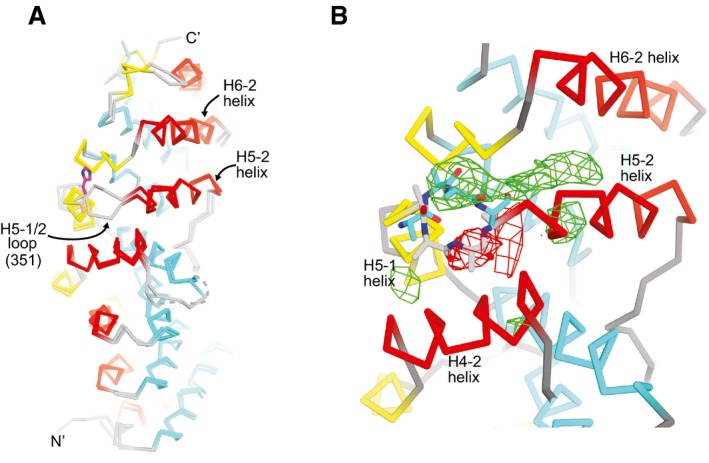
- Cα trace of SPIN90 (351–722) superposed onto the structure of SPIN90 (269–722) from the co‐complex structure. His580 is shown in stick representation. H5‐1/2 is flipped away from the C‐terminus in the inactive 351–722 structure and toward it in the co‐complex structure.
- Zoomed in view from panel (A) showing the difference in conformation of the H5‐1/2 loop in the inactive (351–722, sticks with gray carbon atoms) versus active (269–722, sticks with cyan carbon atoms) SPIN90 structures. Positive (green) or negative (red) Fo−Fc electron density maps are contoured at ±2.0 σ and were generated from the co‐complex reflection file plus a PDB file in which H5‐1/2 loop (residues 577–585) of the co‐complex structure was replaced with the same residues from chain A in the 351–722 structure.
