Figure 4. Contacts of SPIN90 with the long C‐terminal helix (αD) in ARPC4 are important for activation of Arp2/3 complex.
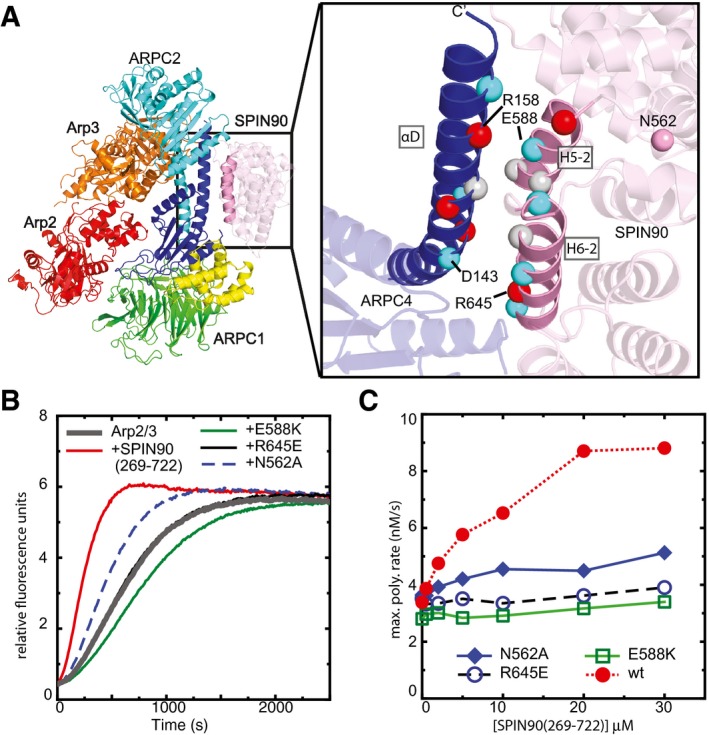
- Ribbon diagram of co‐complex structure with residues at the SPIN90‐Arp2/3 interface depicted as Cα atom spheres colored according to side chain chemistry (gray: hydrophobic, red: basic, blue: acidic). Only the ARPC4 subunit of Arp2/3 complex is shown in the zoomed in panel for clarity.
- Time courses of pyrene actin polymerization showing the influence of mutations on the ability of SPIN90 (269–722) to activate Arp2/3 complex. Reactions contained 3 μM 15% pyrene‐labeled actin, 50 nM Bos taurus Arp2/3 complex, and 20 μM wild‐type or mutant SPIN90 (269–722).
- Plot of maximum polymerization rate versus concentration of SPIN90 (269–722) for reactions as shown in panel (B).
