Abstract
Case summary
A 12-year-old male neutered domestic shorthair cat underwent rhinoscopy due to inspiratory dyspnoea and stertor. Rhinoscopy showed signs of chronic rhinitis and a multinodular nasopharyngeal mucosa. A marked infiltrate of macrophages that contained intracellular parasitic forms morphologically compatible with Leishmania amastigotes were observed on histopathological examination of nasal and nasopharyngeal biopsies. PCR from nasal tissue was positive for Leishmania infantum DNA, confirming the diagnosis of granulomatous rhinitis secondary to this parasite. Two eyelid nodules were identified 2 weeks later. Fine-needle aspiration revealed Leishmania amastigotes within macrophages and in the background. Allopurinol therapy was started, but 5 days later the cat developed dermatological signs compatible with a cutaneous adverse drug reaction. The drug was discontinued and meglumine antimoniate prescribed. Twenty-five days later, the cat presented with acute kidney injury and meglumine antimoniate was discontinued. Despite clinical improvement after fluid therapy, mild azotaemia persisted. The cat was subsequently treated with nucleotides and active hexose correlated compounds (N-AHCC). Four months later upper respiratory signs were exacerbated. A relapse of granulomatous rhinitis was suspected and miltefosine therapy started. Chronic kidney disease (CKD) worsened during miltefosine treatment, having improved under fluid therapy. Since then, the cat has been treated with N-AHCC and renal diet and at the time of writing shows stable CKD with no recurrence of respiratory signs.
Relevance and novel information
This case describes Leishmania infantum as a cause of granulomatous rhinitis in a cat without cutaneous lesions, reporting the alternative use of N-AHCC and miltefosine when allopurinol seemed to have induced a cutaneous rash and there was acute kidney injury (AKI) after meglumine antimoniate therapy.
Keywords: Leishmaniosis, miltefosine, nucleoside-analogues/N-AHCC, Leishmania, granulomatous, rhinitis
Case description
A 12-year-old male neutered domestic shorthair cat was referred in July 2017 to the Internal Medicine service of the Veterinary Teaching Hospital at the Faculty of Veterinary Medicine University of Lisbon for rhinoscopy due to progressive worsening of inspiratory effort with concurrent stertor and episodic sneezing of several weeks’ duration. The cat was indoor-only and was feline immunodeficiency virus (FIV)/feline leukaemia virus (FeLV) negative. The owner reported no history of a cough or nasal discharge. The animal had a normal appetite, without digestive upset or other signs of illness. Body condition score (BCS) was stable for several months (BCS 6/9). Past medical history included recurrent dermatological signs, namely episodic ventral alopecia and pruritic excoriations. Fungal and bacterial cultures were found negative in the past, leading to the suspicion of an allergic disease, ectoparasites or a behavioural condition. Dermatological flare-up episodes had been controlled during the past 5 years by topical corticosteroid treatment and regular spot-on administration of selamectin. A recent biochemical profile performed by the referring veterinarian only revealed a moderate hyperproteinaemia (total proteins 9 g/dl; reference interval 6–8.2 g/dl)].
Physical examination was unremarkable apart from the stertor associated with inspiratory effort. Differential diagnoses included infectious rhinitis, immune-mediated/allergic rhinitis or mechanical obstruction (due to polyp or neoplasia). Oral and nasal swabs were obtained to detect Chlamydia species, feline calicivirus and feline herpesvirus infections by PCR. Rhinoscopy was performed under general anaesthesia (premedication with methadone 0.1 mg/kg IV, induction under propofol 4 mg/kg IV followed by volatile isoflurane maintenance), using a broncho-fibrescope (60001 Storz, working channel 2.3 mm) for retrograde vision and a rigid rhinoscope (Forward-Oblique Telescope 30º Storz) for anterograde inspection. Rhinoscopy showed a mildly hyperaemic nasal mucosa, without turbinate lysis in both nasal cavities. The nasopharynx was not obstructed, but the mucosa showed a multinodular appearance with a mean nodule size of 2–3 mm diameter (Figure 1). The soft palate was moderately thickened. Biopsies were performed from both nasal cavities and nasopharynx tissue, and were submitted to histopathological examination and fungal culture. The animal was discharged under prednisolone therapy owing to the clinical suspicion of chronic rhinitis (0.3 mg/kg for 10 days followed by 0.2 mg/kg for 10 days).
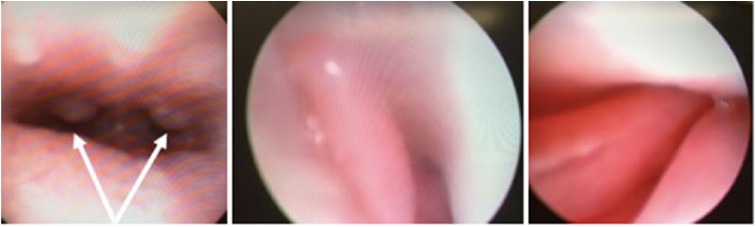
Figure 1.
Rhinoscopy photos showing a non-ulcerated and multinodular nasopharyngeal mucosa (on the left) and mild hyperaemic nasal mucosa (middle photo and on the right). Arrows are showing the nasopharyngeal nodules (mean diameter 2–3 mm)
Molecular tests were negative, as was fungal culture. A marked infiltrate of macrophages that contained intracellular parasitic forms morphologically compatible with Leishmania infantum amastigotes (the agent of zoonotic visceral–cutaneous leishmaniosis) were seen at histopathological evaluation of nasal and nasopharyngeal biopsies (Figure 2). Prednisolone therapy was immediately tapered, and the cat was re-evaluated. Stertor was persistent and two small (2 mm diameter) eyelid nodules in the upper and lower palpebrae of the left eye were identified (Figure 3).
Figure 2.
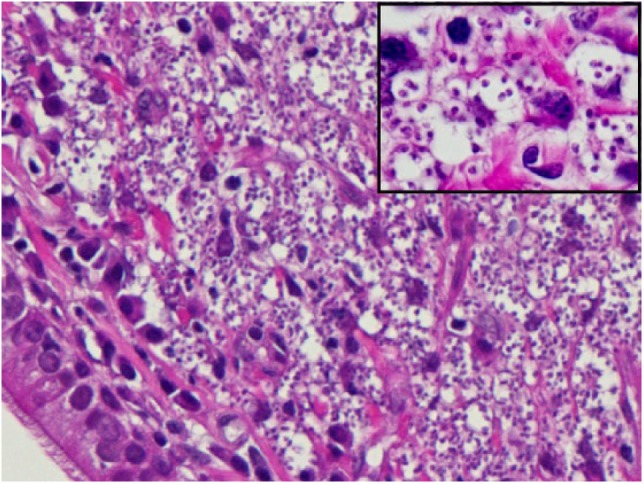
Nasal mucosa biopsy. The lamina propria is packed with large numbers of macrophages filled with Leishmania species. Respiratory epithelium on the lower left (haematoxylin and eosin, × 400). Insert: magnification of the same macrophages with a clearer view of the Leishmania amastigotes (haematoxylin and eosin, × 1000)
Figure 3.

Photos showing bilateral blepharitis with mild periocular alopecia and mild chemosis, bilateral blepharospasm and the presence of small palpebral nodules on the left upper and lower eyelid (arrow)
The cat was anaesthetised in order to complete the investigations. The anaesthetic protocol consisted of premedication with methadone (0.1 mg/kg IV), induction with propofol (2 mg/kg IV), followed by volatile isoflurane maintenance. Fine-needle aspiration (FNA) of palpebral nodules was performed for cytological evaluation and nasal biopsies were repeated for Leishmania PCR. A blood sample was also obtained for complete blood count (CBC), biochemical profile, serum protein electrophoresis, anti-FIV antibody detection (Viracheck FIV; Synbiotics), FeLV DNA and RNA molecular detection (blood real-time PCR), and anti-Leishmania (Qualitative Elisa [IDEXX] and Leishmania Spot IF [Biomerieux]). This last one was validated in previous inter-laboratory assays using 100 feline serum samples, including positive and negative sera from the biobank of Parasitology Lab (Faculty of Veterinary Medicine – University of Lisbon). The cut-off dilution is set as 1/40 and a fluorescently labelled anti-cat IgG conjugate (anti-cat IgG MegaCor; MegaCor Diagnostik) is used. Repeatability and reproducibility were also previously assessed (results not published). A urine sample was obtained for urinalysis and protein: creatinine ratio (UPC). Moreover, thoracic radiographs (left lateral, right lateral and dorsoventral views) and abdominal ultrasound examination were performed.
Splenomegaly was the only observed abnormality. Spleen FNA was suggested but declined by the owner. Leishmania amastigotes filled macrophages and were seen free in the background in FNA smears from eyelid nodules (Figure 4); anti-Leishmania antibodies were detected (Immunofluorescence assay [IFA] titre 320) and Leishmania DNA was identified by PCR from nasal biopsies. Diagnosis of granulomatous rhinitis and palpebral dermatitis caused by Leishmania infection was confirmed. Both FIV and FeLV coinfections were excluded. Hyperproteinaemia, hypergammaglobulinaemia and mild proteinuria (UPC 0.6) were the only clinicopathological abnormalities observed. Urine specific gravity was 1.035. Treatment was initiated with allopurinol 10 mg/kg (Zyloric; FaesFarma) q12h.
Figure 4.
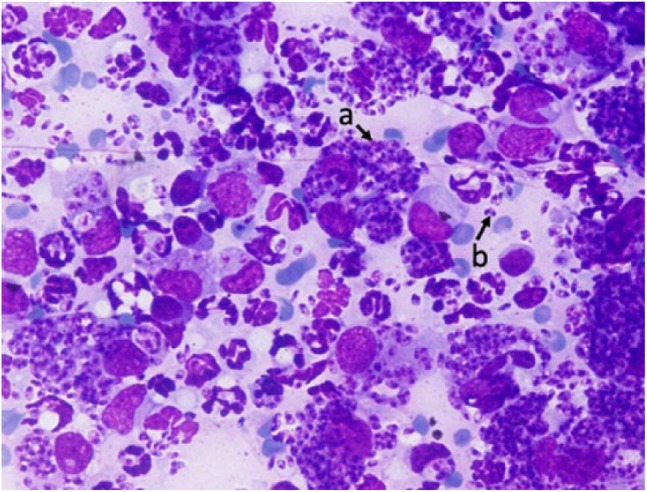
Result of the fine-needle aspirate from an eyelid nodule. The macrophages are filled with Leishmania amastigotes (a), which are also free in the background (b) (Giemsa × 1000)
Five days after the onset of allopurinol, the cat was presented for focal ear, cervical and head alopecia, erythema, exfoliation and crusting (Figure 5). Owing to the short-term therapy, there was no evident improvement of respiratory signs under allopurinol. Fungal culture was negative and an imprint of lesions revealed hyper-segmented neutrophils and some keratinocytes. No Leishmania amastigotes were observed. A cutaneous adverse drug reaction (CADR) was strongly suspected. Allopurinol was stopped and 4 days later dermatological lesions spontaneously resolved.
Figure 5.
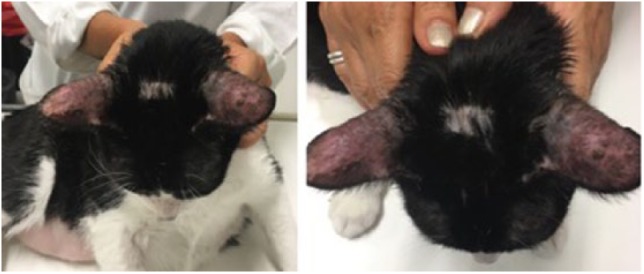
Erythema and alopecia on outer surface of the pinnae manifested some days after starting allopurinol treatment. Focal alopecia is also evident on the head
In lieu of allopurinol, the cat was prescribed meglumine antimoniate (Glucantime 50 mg/kg SC q24h; Boehringer Ingelheim Animal Health), and both renal function and protein profile were monitored 10 and 25 days later (Table 1). A moderate improvement in respiratory signs was observed 1 week after the onset of this therapy.
Table 1.
Timeline showing variations of creatinine, urea, urinary protein: creatinine ratio (UPC), urine specific gravity (USG), total proteins (TP), albumin (Alb) and the prescribed medical treatment
| Time points/medical treatment | Creatinine mg/dl (RI 0.8–1.6) |
Urea mg/dl (RI 30–60) |
UPC (<0.2) |
USG | TP g/dl (RI 6–8.2) |
Alb g/dl (RI 2.5–3.9) |
|---|---|---|---|---|---|---|
| Diagnosis – Started on allopurinol 10 mg/kg PO q12h – Allopurinol stopped 5 days later owing to a suspicion of CADR |
0.9 | 62.5 | 0.6 | 1.035 | 9 | 1.5 |
| Day 0 – Started on meglumine antimoniate 50 mg/kg SC q24h |
1.2 | 75.9 | 0.6 | 1.035 | 9.6 | 2.5 |
| Day 10 – Meglumine antimoniate was continued |
1.2 | 20 | * | 1.025 | 9.6 | 2.7 |
| Day 25 – Meglumine antimoniate was discontinued – Hospitalisation for fluid therapy, supportive and ocular treatment |
6.5 | 119 | * | 1.030 | 9.1 | 2.8 |
| Day 34 – Hospital discharge |
0.9 | 31.4 | * | * | * | * |
| Day 44 – Started on N-AHCC ½ tablet PO q24h + benazepril 0.5 mg/kg PO q24h |
1.8 | 117.8 | 0.4 | 1.030 | 7.58 | 3.59 |
| Day 123 – N-AHCC + benazepril were continued |
1.89 | 93.4 | 0.3 | 1.030 | 8.8 | 3.0 |
| Day 162 – Started on miltefosine 2 mg/kg PO q24h for 28 days; N-AHCC + benazepril were continued |
1.9 | 83 | 0.3 | 1.028 | 8.8 | 2.9 |
| Day 180 – Hospitalisation for fluid therapy and supportive treatment. Miltefosine and N-AHCC were continued; benazepril was discontinued |
3.84 | 141.9 | * | * | 9.35 | 2.96 |
| Day 188 – Hospital discharge |
1.96 | 103.8 | <0.2 | * | * | * |
| Day 204 – N-AHCC, renal diet, SC regular fluid therapy, intermittent administration of maropitant |
2.33 | 120.6 | <0.2 | 1.028 | * | * |
| Day 213 – Same medical therapy and diet |
3.28 | 198.9 | * | 1.025 | 8.19 | 3.1 |
| Day 268 – Same medical therapy and diet |
3.67 | 138.1 | * | 1.028 | 8.57 | 3.16 |
| Day 315 – Same medical therapy and diet |
3.31 | 115 | <0.2 | 1.025 | 8.2 | 3.14 |
RI = reference interval; CADR = cutaneous adverse drug reaction; N-AHCC = nucleotides and active hexose correlated compounds
refers to missing data at each time-point
On day 25, the cat was presented for lethargy and progressive anorexia. At physical examination only a unilateral blepharospasm in the left eye was observed. Ophthalmological examination revealed the presence of a conjunctival oedema, deposition of fibrin in the anterior chamber and decreased intraocular pressure. These findings were consistent with anterior uveitis. Even if other systemic causes could have contributed to the uveitis (eg, neoplasia or concurrent infections), the previous medical exploration did not reveal the presence of viral infections or significant changes on thoracic radiographs and abdominal ultrasound. Therefore, uveitis was assumed to be a consequence of leishmaniosis. Biochemical results confirmed the presence of a severe azotaemia (day 25; Table 1). CBC showed a neutrophilic non-specific leukocytosis (26.110/μl). Urine culture was negative. The cat was hospitalised for intravenous fluids (Ringer’s lactate with potassium chloride maintenance supplementation) and supportive care with maropitant (Cerenia 1 mg/kg q24h).
Treatment of uveitis consisted of topical dexamethasone (q6h), flurbiprofen (q6h) and tropicamide (q12h) until progressive remission of ocular signs 2 weeks later. Meglumine antimoniate was discontinued. Appetite and azotaemia normalised, and the cat was discharged 9 days later (day 34; Table 1). A renal diet (Purina N/F mixed dry and canned) was started. The animal was evaluated 10 days later (day 44; Table 1) without any report of adverse events or changes on physical examination. Chronic kidney disease (CKD) was classified according to IRIS on stage 2 with borderline proteinuria (UPC 0.4) and normotensive systolic blood pressure (160 mmHg). Owing to the previous history of CADR under allopurinol and the suspicion of acute kidney injury (AKI) secondary to meglumine antimoniate therapy, a dietary supplement of nucleotides and active hexose correlated compounds (N-AHCC) was started (Impromune ½ tablet once daily; Bioiberica). Owing to borderline proteinuria, benazepril (Fortekor 0.5 mg/kg q24h) was also added.
Six months after first examination (day 123; Table 1), the cat condition was stable with stage 2 IRIS CKD, borderline proteinuria; it was non-hypertensive and had stable antibody titres (IFA titre 160).
Two months later (day 162; Table 1) the cat was re assessed because of recurrence of sneezing and stertor. Azotaemia, hyperproteinaemia, and anti-Leishmania antibody titre were stable (IFA titre 320). The owner declined further investigations to confirm the recurrence of granulomatous rhinitis. Considering the previous CADRs to allopurinol and AKI, and probably secondary meglumine antimoniate, extra-label miltefosine (Milteforan) was started at the current dosage used for dogs (2 mg/kg PO q24h). A few vomiting episodes were reported in the first week of therapy but were transient and managed by using maropitant (Cerenia). Respiratory signs rapidly improved during the first week of treatment.
The cat was presented for follow-up examination 3 weeks later (day 180; Table 1) showing a decreased appetite and an increase in azotaemia. These findings justified a return to hospitalisation for fluid therapy and supportive care as previously performed. An acute-on-chronic episode of CKD was suspected, either worsened by miltefosine administration or due to the progression of the disease. During hospitalisation, azotaemia progressively decreased and miltefosine treatment was completed (28 days) under fluid therapy.
The cat was discharged 1 week later (day 188; Table 1). Benazepril had been discontinued during hospitalisation and was not reintroduced owing to the fact that proteinuria was negative on multiple further controls (UPC <0.2). Since discharge, and at the time of writing, the cat has been routinely monitored with physical examination and renal profile every 4–6 weeks (Table 1). No recurrence of skin, respiratory, ocular or mucosal signs has been observed and body weight has remained stable. None-theless, azotaemia has been progressively worsening (Table 1). At the last check (day 315; Table 1), 10 months after diagnosis, CKD was staged according to IRIS classification as stage 3/4, and the cat was non-proteinuric and non-hypertensive. Current therapy consists of N-AHCC, renal diet, regular subcutaneous fluid therapy and intermittent administration of maropitant (1 m/kg PO q24h).
Discussion
This case report not only shows an unusual presentation of feline leishmaniosis, but also describes suspected side effects of drugs currently used for its medical management. Although hypergammaglobulinaemia and mucocutaneous lesions are frequently found among infected cats, respiratory signs are particularly rare.1–7 Chronic nasal discharge and dyspnoea are uncommon and reported to occur in <25% of infected cats.3,8 In this case, the primary presenting complaint was stertor and inspiratory effort.
A diagnosis of leishmaniosis was confirmed after identification of granulomatous rhinitis with concurrent intracellular amastigotes in the histopathological analysis of nasal and nasopharyngeal biopsies, as well as direct observation of Leishmania on FNA of palpebral nodules. Palpebral nodules were not present on initial examination but only became evident at recheck, probably owing to a brief use of prednisolone, which may have resulted in mild immuno suppression. Additional tests that supported a diagnosis of leishmaniosis were serological (IFA and ELISA) and PCR from nasal tissue. Furthermore, clinical response to therapy and consequent regression of clinical signs also strengthened this diagnosis. Concurrent causes for immunosuppression such as retroviral infections were investigated but found to be negative. As in the majority of cases of feline leishmaniosis, evidence of concurrent immunosuppression, other than the brief use of topical prednisolone during flare-up dermatological episodes, was not identified.1,3
Because there are no controlled studies about feline leishmaniosis treatment, allopurinol was prescribed in this cat as this compound has been anecdotally proven to be successful.1–3,9–11 However, the acute presentation of dermatological signs 5 days after onset of this therapy led to the suspicion of allopurinol CADR. Adverse reactions to this drug have been described in humans undertaking allopurinol,12–14 but to our knowledge, it has not been previously reported in cats. This dermatological reaction is believed to be due to a specific T-cell response activated by oxypurinol, one of the metabolites of allopurinol.15 Despite the absence of histopathological analysis, this reaction was strongly suspected owing to the fact that it happened several days after the onset of therapy and dermatological signs resolved after stopping allopurinol therapy.
Consequently, the therapeutic strategy was modified and meglumine antimoniate was started. Meglumine antimoniate is known to be potentially nephrotoxic in dogs;16 however, information about its side effects is still scarce. In this cat, an AKI episode was observed after 17 days. As there was no azotaemia before the onset of meglumine antimoniate therapy, it is possible that this compound led to tubular renal injury, as has been described in dogs.16 However, it is also possible that CKD was present, but silent (IRIS stage 1), in this geriatric patient and azotaemia progressively worsened after therapy. Use of the symmetric dimethylarginine assay may have enhanced our assessment of renal function before the onset of meglumine antimoniate therapy. This was not performed as local constraints (unavailability of this parameter in that period and financial issues) were a limitation in this case. Meglumine antimoniate was discontinued and azotaemia improved after IV fluid therapy. Nonetheless, there was a persistent mild azotaemia. A diagnosis of CKD was concurrently established, and therapy was adapted according to IRIS recommendations.
Recently, N-AHCC have been considered an alternative maintenance therapy to allopurinol (following meglumine antimoniate in dogs) showing similar activity against Leishmania species.17 To our knowledge, this is the first published study detailing the use of N-AHCC in a cat. Treatment did not worsen clinical signs or result in adverse side effects. Indeed, the cat was stable for 120 days. A few weeks after medical stabilisation (120 days after the start of N-AHCC and 140 days after stopping meglumine antimoniate therapy), respiratory signs recurred. It is unclear whether this improvement and concurrent relapse was due to the temporary effect of meglumine antimoniate and prolonged N-AHCC therapy or, from another perspective, possible N-AHCC treatment failure. Further studies are needed to assess the truly efficacy of N-AHCC in feline patients. As medical exploration was declined, a relapse of granulomatous rhinitis secondary to leishmaniosis was assumed. This was further supported by the good clinical response and improvement of respiratory signs under miltefosine.
Miltefosine is a well-known drug, currently used in human and canine leishmaniosis.18–22 Information concerning its use in cats is particularly scarce. A recent study has described hyporexia and weight loss as the main adverse effects of miltefosine therapy (2 mg/kg PO q24h) in feline refractory sporotrichosis.23 To our knowledge, only one case of feline leishmaniosis treated with miltefosine has been reported in the literature.24
Recognising the risk of unexpected side effects, use of this leishmanicidal therapy was started off label, using the same dosage currently recommended for dogs.7 However, azotaemia worsened during treatment. In humans, miltefosine-induced nephrotoxicity has been described in 29% of patients.13 In dogs, administration of this compound does not seem to induce renal lesions.16 As literature on this subject is scarce, possible miltefosine-induced CKD is possible in this scenario. However, an acute-on-chronic azotaemia unrelated to miltefosine or eventually worsened by the transient vomiting cannot be excluded. Considering the pros and cons of stopping this compound and recognising that there were no other alternatives for leishmaniosis management, miltefosine therapy was continued but under IV fluids. Despite the fact that CKD progressively worsened, the cat, at the time of writing, is stable (10 months after diagnosis) under medical therapy and following IRIS recommendations.
Conclusions
This case reinforces the idea that granulomatous rhinitis secondary to leishmaniosis should be included in the differential diagnoses of obstructive chronic rhinitis in cats, particularly in endemic areas. This case also explores the difficulties of medical management of this disease in feline patients, describing the alternative use of miltefosine and N-AHCC in a cat, when allopurinol appeared to have induced a cutaneous rash and there was AKI after meglumine antimoniate therapy.
Acknowledgments
We would like to thank Professor Solange Gil, and the medical and nurse staff of the Veterinary Teaching Hospital – Faculty of Veterinary Medicine, University of Lisbon, for the care provided to this cat during hospitalisation and follow-up. We also thank Dr Ryane Englar for reviewing the manuscript and Lidia Gomes for the technical support.
Footnotes
Accepted: 15 October 2018
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this work was provided by Centre for Interdisciplinary Research in Animal Health (CIISA) through project UID/CVT/276/2013.
ORCID iD: Rodolfo Oliveira Leal  https://orcid.org/0000-0002-2463-4062
https://orcid.org/0000-0002-2463-4062
Esmeralda Delgado  https://orcid.org/0000-0001-9383-1310
https://orcid.org/0000-0001-9383-1310
References
- 1. Pennisi MG, Hartmann K, Lloret A, et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pennisi MG, Venza M, Reale S, et al. Case report of leishmaniasis in four cats. Vet Res Commun 2004; 28: 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pennisi M-G, Cardoso L, Baneth G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors 2015; 8: 302. DOI: 10.1186/s13071-015-0909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basso MA, Marques C, Santos M, et al. Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. JFMS Open Rep 2016; 2. DOI: 10.1177/2055116916630002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Navarro JA, Sánchez J, Peñafiel-Verdú C, et al. Histopathological lesions in 15 cats with leishmaniosis. J Comp Pathol 2010; 143: 297–302. [DOI] [PubMed] [Google Scholar]
- 6. Ibba F. Un caso di rinite cronica in corso di leishmaniosi felina. Proc 62nd Int SCIVAC Congr Rimini Soc Cult Ital Vet Anim Compagnia; 2009. May 29–31; Rimini, Italy: Zoomark International. [Google Scholar]
- 7. Pennisi MG, Persichetti MF. Feline leishmaniosis: is the cat a small dog? Vet Parasitol 2018; 251: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altuzarra R, Movilla R, Roura X, et al. Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet Radiol Ultrasound Epub ahead of print 11 July 2018. DOI: 10.1111/vru.12666. [DOI] [PubMed] [Google Scholar]
- 9. Maia C, Sousa C, Ramos C, et al. First case of feline leishmaniosis caused by Leishmania infantum genotype E in a cat with a concurrent nasal squamous cell carcinoma. JFMS Open Rep 2015; 1. DOI: 10.1177/2055116915593969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leiva M, Lloret A, Peña T, et al. Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol 2005; 8: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rüfenacht S, Sager H, Müller N, et al. Two cases of feline leishmaniosis in Switzerland. Vet Rec 2005; 156: 542–545. [DOI] [PubMed] [Google Scholar]
- 12. Halevy S, Ghislain P-D, Mockenhaupt M, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol 2008; 58: 25–32. [DOI] [PubMed] [Google Scholar]
- 13. Ranu H, Jiang J, Ming PS. A case series of allopurinol-induced toxic epidermal necrolysis. Indian J Dermatol 2011; 56: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atzori L, Pinna AL, Mantovani L, et al. Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department – Cagliari University (Italy). J Eur Acad Dermatol Venereol 2012; 26: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 15. Yun J, Mattsson J, Schnyder K, et al. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin Exp Allergy 2013; 43: 1246–1255. [DOI] [PubMed] [Google Scholar]
- 16. Bianciardi P, Brovida C, Valente M, et al. Administration of miltefosine and meglumine antimoniate in healthy dogs: clinicopathological evaluation of the impact on the kidneys. Toxicol Pathol 2009; 37: 770–775. [DOI] [PubMed] [Google Scholar]
- 17. Segarra S, Miró G, Montoya A, et al. Randomized, allopurinol-controlled trial of the effects of dietary nucleotides and active hexose correlated compound in the treatment of canine leishmaniosis. Vet Parasitol 2017; 239: 50–56. [DOI] [PubMed] [Google Scholar]
- 18. Sundar S, Olliaro PL. Miltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk management. Ther Clin Risk Manag 2007; 3: 733–740. [PMC free article] [PubMed] [Google Scholar]
- 19. Solano-Gallego L, Miró G, Koutinas A, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 2011; 4: 86. DOI:10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miró G, Oliva G, Cruz I, et al. Multicentric, controlled clinical study to evaluate effectiveness and safety of miltefosine and allopurinol for canine leishmaniosis. Vet Dermatol 2009; 20: 397–404. [DOI] [PubMed] [Google Scholar]
- 21. Manna L, Vitale F, Reale S, et al. Study of efficacy of miltefosine and allopurinol in dogs with leishmaniosis. Vet J 1997 2009; 182: 441–445. [DOI] [PubMed] [Google Scholar]
- 22. Manna L, Corso R, Galiero G, et al. Long-term follow-up of dogs with leishmaniosis treated with meglumine antimoniate plus allopurinol versus miltefosine plus allopurinol. Parasit Vectors 2015; 8: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silva FDS, Cunha SC, dos S, Baptista AR, de S, et al. Miltefosine administration in cats with refractory sporotrichosis. Acta Sci Vet 2018; 46: 1571 DOI: 10.22456/1679-9216.83639. [DOI] [Google Scholar]
- 24. Bardagi M, Lloret A, Dalmau A, et al. Feline Leishmaniosis: 15 cases. Vet Dermatol 2016; 27 Suppl 1: 112–113. [Google Scholar]