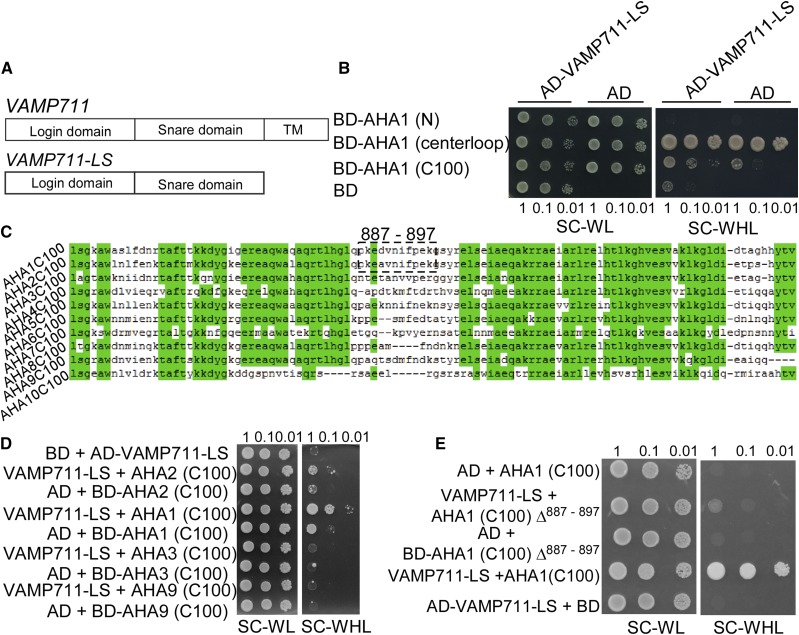
Figure 2.
VAMP711 interacts with the C terminus of AHA1 and AHA2. A, Schematic structure of VAMP711 protein structure: longin domain, SNARE domain, and transmembrane domain (TMD). The longin and SNARE domains of VAMP711 (VAMP711-LS) were used in a yeast two-hybrid assay. B, Yeast two-hybrid assay to detect the interaction among the AHA1 N terminus, centerloop, C terminus, and VAMP711. C, The sequence alignment analysis of the C terminus of PM H+-ATPase (AHA) in Arabidopsis. D, Yeast two-hybrid assay to detect the interaction of VAMP711 with the C terminus of AHA family members. E, Yeast two-hybrid assay to detect the region of interaction between AHA1 C terminus, AHA1 C terminus minus amino acid residues 887 to 897 (AHA1 (C100)Δ887–897), and VAMP711. Yeast strains expressing the indicated plasmids were grown on synthetic complete medium without Trp and Leu (SC-WL, left) and on synthetic complete medium without Trp, Leu, and His (SC-WHL, right). Photographs were taken after 3 to 5 d of growth on the indicated medium. Panels show yeast serial decimal dilutions. Experimental details are provided in the “Materials and Methods.”