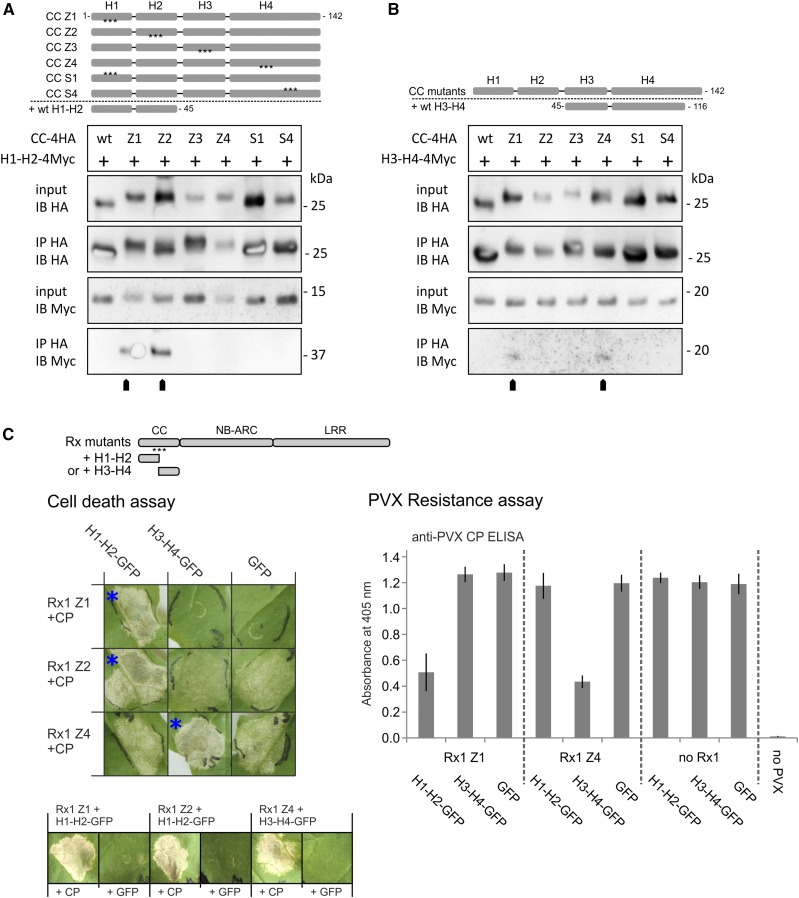
Figure 4.
A and B, Immunoprecipitation assay to test if the loss of interaction between H1-H2 and H3-H4 due to mutations (Z1–Z4, S1, and S4) causes the strands to dissociate in the complete CC and expose internal binding surfaces. HA-tagged versions of the wild-type (wt) and mutant Rx1 CC (amino acids 1–142) were coexpressed with wild-type H1-H2-4Myc (amino acids 1–45; A) or H3-H4-4Myc (amino acids 45–116; B), as indicated by schematic overviews of the constructs. The CC constructs were immunoprecipitated with antibodies against the HA tag. Coimmunoprecipitation of the interacting H1-H2 or H3-H4 strands was detected by anti-Myc immunoblotting. C, Complementation of the loss of function caused by the Z1, Z2, and Z4 mutations via coexpression of wild-type H1-H2 or H3-H4 strands. Full-length Rx1 mutant constructs displaying decreased elicitor-dependent cell death (Z1, Z2, and Z4) or decreased PVX resistance (Z1 and Z4) were coexpressed with H1-H2-GFP, H3-H4-GFP, or GFP to investigate if the presence of the wild-type strands could restore the functionality of Rx1. Combinations in which Rx1-mediated cell death was reconstituted are indicated by blue asterisks. These three combinations also were tested in the absence of the CP to determine if the coexpressed CC fragment induces an autoactive response (row of images at bottom). Resistance was assessed by the detection of PVX in an ELISA (error bars represent the sd; n = 8). The CC strands or GFP were coexpressed with PVX:GFP in the absence of Rx1 as a negative control.