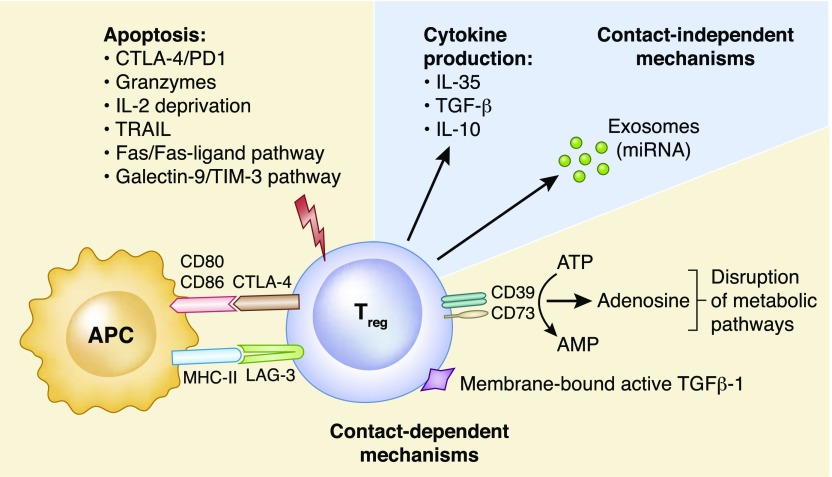
Figure 1.
Mechanisms of Treg suppression. Contact-dependent mechanisms: Induction of apoptosis via engagement of cytotoxic T lymphocyte antigen-4 (CTLA-4) and programed cell death 1 (PD1), granzyme A/B, TNF-related apoptosis-inducing ligand (TRAIL), the Fas/Fas-ligand pathway, the galectin-9/T cell immunoglobulin and mucin domain-3 (TIM-3) pathway and through IL-2 deprivation. Interaction of CTLA-4 with its ligand CD80/86 on antigen presenting cells (APCs) delivers a negative signal that prevents T cell activation. Cell surface lymphocyte activation gene 3 (LAG-3) binds to MHC class II molecules preventing the maturation and the ability of APCs to activate effector T cells. Expression of membrane-bound active TGFβ-1. Disruption of metabolic pathways through the production of adenosine from the ectoenzymes CD39/CD73-mediated degradation of extracellular ATP to AMP. ATP-derived adenosine increases intracellular cAMP levels on Tregs that is transferred to T effector cells through gap junctions, leading to the upregulation of inducible cAMP early repressor (ICER) and in turn, the inhibition of nuclear factor of activated T-cells (NFAT) and IL-2 transcription. Contact-independent mechanisms: Anti-inflammatory cytokine production (IL-10, IL-35, and TGF-β) has been linked with inhibition of T cell activation in vivo. Transfer of micro ribonucleic acid (miRNA) through exosomes can silence specific genes in T effector cells, preventing proliferation and cytokine production.