Figure 4. Divergent actions on β-arrestin2 recruitment by OBP versus EBP mutations.
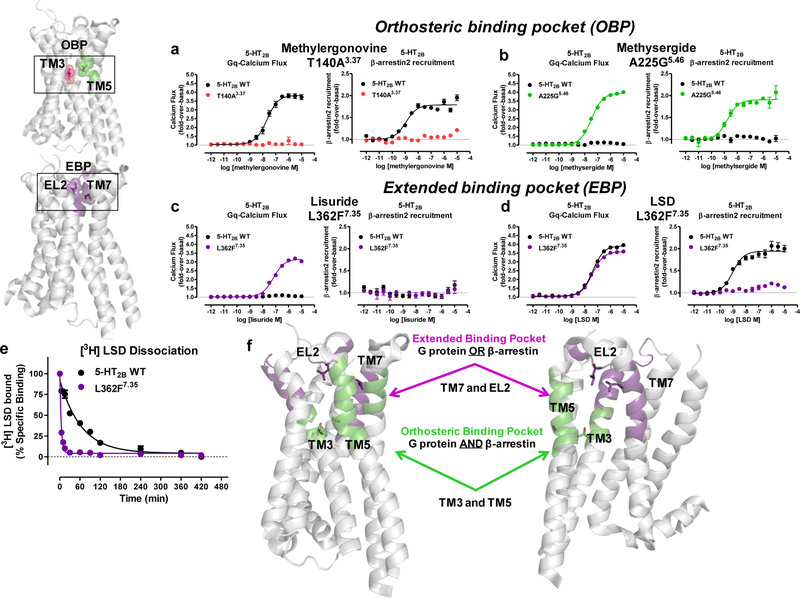
Examination of β-arrestin2 recruitment activity at OBP T140A3.37 and A225G5.46 mutations versus EBP mutation L362F7.35. a) Methylergonovine Gq-mediated calcium flux (left panel) comparing T140A3.37 (red) to 5-HT2BR WT (black, EC50 = 21 nM). β-arrestin2 recruitment (right panel) comparing T140A3.37 (red) to 5-HT2BR WT (black, EC50 = 1.2 nM). b) Methysergide Gq-mediated calcium flux (left panel) comparing A225G5.46 (green, EC50 = 33 nM) to 5-HT2BR WT (black). β-arrestin2 recruitment (right panel) comparing A225G5.46 (green, EC50 = 1.7 nM) to 5-HT2BR WT (black). c) Lisuride Gq-mediated calcium flux (left panel) comparing L362F7.35 (purple, EC50 = 65 nM) to 5-HT2BR WT (black). β-arrestin2 recruitment (right panel) comparing L362F7.35 (purple) to 5-HT2BR WT (black). d) LSD Gq-mediated calcium flux (left panel) comparing L362F7.35 (purple, EC50 = 40 nM) to 5-HT2BR WT (black, EC50 = 42 nM). β-arrestin2 recruitment (right panel) comparing L362F7.35 (purple) to 5-HT2BR WT (black, EC50 = 0.97 nM). Data in panels a-d are expressed as fold-over-basal and represent mean and S.E.M from three independent experiments (N=3) performed in triplicate. e) LSD dissociation comparing 5-HT2BR wild-type (black, koff = 0.015 min−1) to the L362F7.35 mutant (purple, koff = 0.240 min−1). Data represent percent specific binding indicating mean and S.E.M from three independent experiments (N=3) performed in duplicate. f) Schematic comparing the location of the EBP residues L209EL2 and L3627.35 (purple), which can result in either Gq or β-arrestin2 recruitment preference, to the location of OBP residues T1403.37 and A2255.46 (green), which result in equal contributions to Gq activity and β-arrestin2 recruitment.
