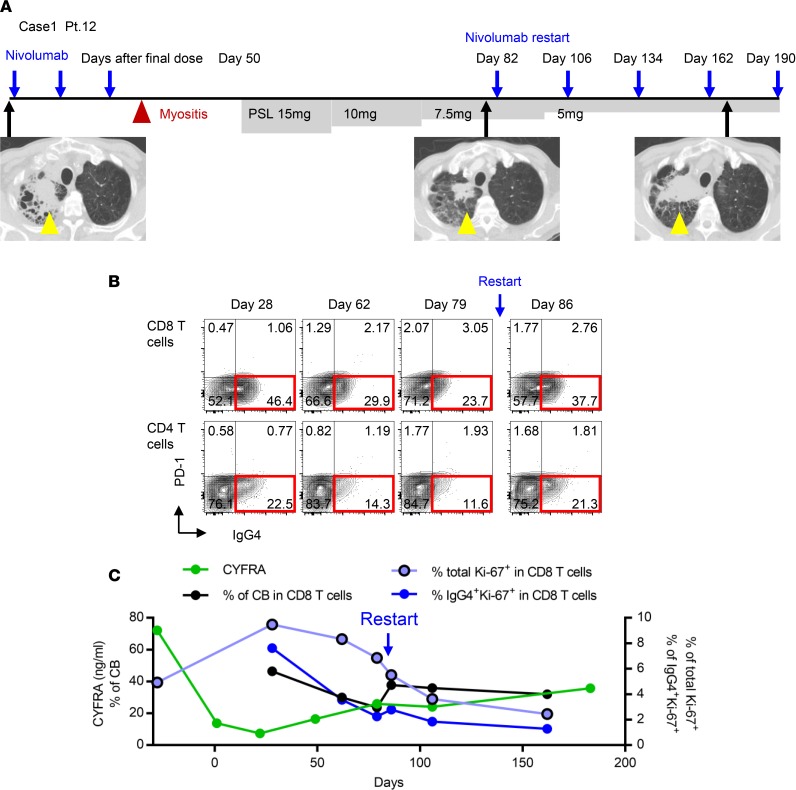
Figure 7. Clinical applications of monitoring of nivolumab binding and Ki-67 positivity in T cells to determine residual efficacy in NSCLC patients.
(A) Case 1 (Pt. 12): Although the tumor initially responded to nivolumab, treatment was discontinued after 3 doses due to myositis and then restarted after 82 days off. Yellow triangles show tumor in right lung. PSL, prednisolone. (B) Nivolumab-binding status in CD8 and CD4 T cells at the indicated time points. (C) Tumor marker, CYFRA (ng/ml); percentage of CB of nivolumab in CD8 T cells; and percentage of Ki-67+ cells in total and nivolumab-bound CD8 T cells were followed up.