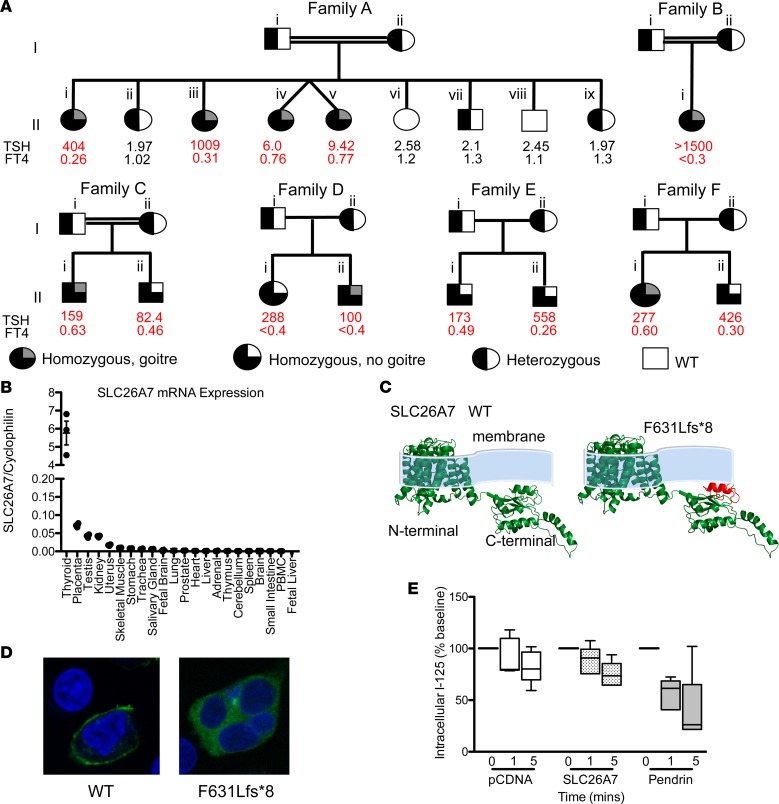
Figure 2. Clinical genotype and phenotype data for kindreds harboring SLC26A7 mutations, and functional characterization of wild-type and mutant SLC26A7.
(A) Pedigrees of 6 kindreds illustrating cases with congenital hypothyroidism (CH) and unaffected relatives for whom genetic data were available. Thyroid hormone measurements refer to venous measurements made at diagnosis of CH or following genetic evaluation. Reference ranges: AII.i, iii, iv, v thyroid-stimulating hormone (TSH) 0.9–3.5 mU/l, free thyroxine (FT4) 1.1–1.8 ng/dl; AII.ii TSH 0.6–4.8 mU/l, FT4 0.76–1.5 ng/dl; AII.vi. vii, viii, ix TSH 0.4–3.5 mU/l, FT4 0.83–1.69 ng/dl; BII.i TSH 0.7–5.3 mU/l, FT4 1.1–1.8 ng/dl; CII.i TSH 0.6–4.8 mU/l, FT4 0.8–1.5 ng/dl; CII.ii TSH 0.4–3.5 mU/l, FT4 0.8–1.9 ng/dl; DII.i, ii, EII.i, ii, FII.i, ii TSH 0.35–40 mU/l, FT4 0.7–1.5 ng/dl. Double horizontal lines indicate consanguinity. FT4: multiply by 12.87 to convert to pmol/l. (B) Quantitative RT-PCR analysis of SLC26A7 mRNA expression in a human tissue library relative to cyclophilin; horizontal bars denote mean and vertical bars standard error of the mean. (C) Homology modeling predicting the protein consequences of the SLC26A7 p.F631Lfs*8 mutation. The deleted protein region is shown in red. (D) HEK293 cells transfected with GFP-tagged wild-type and F631Lfs*8 SLC26A7 demonstrate localization of wild-type protein to the plasma membrane, whereas the mutant remains intracellular (total original magnification, ×63). (E) Box-and-whisker plots for time-dependent iodide efflux from HEK293 cells cotransfected with NIS and pCDNA (white), pendrin (gray), or SLC26A7 (stippled black); n = 5 experiments. Boxes extend from the 25th to 75th percentiles, the horizontal line represents the median, and the vertical bars represent the minimum and maximum values.