Abstract
Diabetic kidney disease (DKD) is the leading cause of end-stage renal failure in the western world. Current treatment of diabetic kidney disease relies on nutritional management and drug therapies to achieve metabolic control. Here, we discuss the potential application of hyperbaric oxygen therapy (HBOT) for the treatment of diabetic kidney disease (DKD), a treatment which requires patients to breathe in 100% oxygen at elevated ambient pressures. HBOT has traditionally been used to diabetic foot ulcers (DFU) refractory to conventional medical treatments. Successful clinic responses seen in the DFU provide the underlying therapeutic rationale for testing HBOT in the setting of DKD. Both the DFU and DKD have microvascular endothelial disease as a common underlying pathologic feature. Supporting evidence for HBOT of DKD comes from previous animal studies and from our preliminary prospective clinical trial reported here. We report urinary metabolomic data obtained from patients undergoing HBOT for DFU, before and after exposure to 6 weeks of HBOT. The preliminary data support the concept that HBOT can reduce biomarkers of renal injury, oxidant stress, and mitochondrial dysfunction in patients receiving HBOT for DFU. Further studies are needed to confirm these initial findings and correlate them with simultaneous measures of renal function. HBOT is a safe and effective treatment for DFU and could also be for individuals with DKD.
Keywords: Diabetic kidney disease, Hyperbaric oxygen therapy, Biomarkers, Reactive oxygen species, Heme oxygenase, Mitochondrial function, Chronic kidney disease
Introduction
In this paper, we explore a potentially novel treatment for diabetic kidney disease (DKD), a major cause of end-stage renal disease and death in individuals with diabetes mellitus (DM) (Toth-manikowski and Atta 2015, United States Renal Data System, 2017). The treatment, hyperbaric oxygen therapy (HBOT), is a systemic therapy that has already been shown to be effective in treatment of the diabetic foot ulcer (DFU), a common limb and life-threatening complication of DM. Here, we present the therapeutic rationale for HBOT of DKD and provide preclinical and first-in-human data to support this hypothesis.
The International Diabetes Federation estimates that 46 million people in North America and 425 million people worldwide are living with diabetes as of 2017 (International Diabetes Federation 2017). These numbers are expected to increase by 35% and 48%, respectively by the year 2045. Of the 425 million people currently living with diabetes, 327 million (76.9%) are between the ages of 20 and 64 years old. Type 2 diabetes accounts for nearly 90% of all cases in high-income countries including the USA (Gallagher and Suckling 2016). The diabetic state results from a combination of inadequate insulin production and a resistance by peripheral tissues to the effect of insulin (insulin resistance—IR) leading to pathologically elevated levels of blood glucose. IR may precede DM, a condition known as the metabolic syndrome. Both IR and the metabolic syndrome are linked to obesity. So tightly linked are these pathologic states that some refer to a modern “diabesity” epidemic (Kalra 2013). These metabolic abnormalities are characterized by systemic chronic inflammation and oxidant stress which over time, lead to endothelial cell damage and systemic vascular disease. All major complications of DM—retinopathy, ischemic cardiomyopathy, DKD, and DFU are the direct result of this diabetic vasculopathy and this is driven by chronic hyperglycemia (International Diabetes Federation 2017).
Chronic kidney disease (CKD) is defined as the gradual decline in kidney function (glomerular filtration rate—GFR) over time, affecting nearly 13% of adults (Darshi et al. 2016; Gallagher and Suckling 2016). The leading cause of renal failure or end-stage renal disease, (ESRD, stage V CKD) in the western world is DKD. This is due to the high prevalence of type 2 diabetes mellitus (T2DM) and because T2DM is six times more likely to lead to DKD compared to non-diabetic individuals (Flyvbjerg 2000; Altemtam et al. 2012; Gallagher and Suckling 2016). A pathologic hallmark of DKD is the presence of increased loss of proteins, especially albumin through the renal filtration membrane (glomerulus). The glomerulus is made up of microvasculature and when damaged by years of hyperglycemia, it begins to leak albumin into the urine, a condition known as albuminuria. The presence of DKD is considered to be the best predictor of mortality in patients with DM (Toth-manikowski and Atta 2015).
Hyperglycemia is a precursor to the development of DKD. This was first elucidated in 2001 by Brownlee when he described that hyperglycemia leads to increased glycolysis, which in turn upregulates several different pathways (Brownlee 2001). The pathogenesis due to hyperglycemia results from an increasing number of non-enzyme-related glycosylation of proteins, with the resultant production of the pathologic species, so-called advanced glycation end products (AGE), and a concomitant pathologic activation of protein kinase C as well as the hexosamine and polyol pathways. These downstream biochemical effects of hyperglycemia can induce chronic oxidative stress and the prolonged activation of several pathologic signal transduction pathways within kidney tissues and systemically. This vascular and tissue damage then leads to the release of pro-inflammatory and pro-fibrotic mediators (Gallagher and Suckling 2016). Hyperglycemia activates the pro-inflammatory transcription factor NF-κB. Because NF-κB is localized to glomerular, interstitial, and tubular epithelial cells in kidneys, hyperglycemia will directly increase its activity. Increased NF-κB levels are correlated with proteinuria and interstitial inflammatory cell filtration resulting in a positive feedback loop, perpetuating and even worsening albuminuria, and declining GFR. Furthermore, it is recognized that cytokines such as TNF and several interleukins are present in higher amounts in diabetic kidneys reflecting the elevated inflammatory state and a close association with increased urinary albumin excretion. This inflammation can induce apoptosis of epithelial cells, which is additionally toxic for renal cells (Toth-manikowski and Atta 2015). Hyperglycemia also initiates the production and accumulation of glycated biomolecules (Negre-Salvayre et al. 2009). These glycation reactions can occur simultaneously with the generation of ROS in a process called glycoxidation. The damage caused by glycoxidation is greater than the damage caused by either oxidation or glycation alone (Islam et al. 2017).
Current treatment of DKD relies on the same basic metabolic therapy used for diabetes in general which includes blood pressure control, nutritional management, exercise, weight loss, and drug therapy. Reduction of sugar consumption helps to reduce general symptoms of diabetes, and salt intake is additionally important for DKD management as less salt in the system will reduce blood pressure in DKD patients. Increased salt intake increases blood pressure, which can cause kidney damage due to the higher pressure of the blood flowing through the kidneys. Angiotensin-converting enzyme (ACE) inhibitors are often used in combination with angiotensin receptor blocker (ARB) inhibitors to decrease the risk of developing end-stage renal failure as they have a synergistic effect on blood pressure and renin release to reduce proteinuria (Gallagher and Suckling 2016). In addition, other treatments have been pursued to target different aspects of DKD progression. These include metabolic inhibitors such as aldose reductase inhibitors (epalrestat), advanced glycosylation end product inhibitors (pyridoxamine), hexosamine pathway inhibitors (azaserine), and inhibitors of NF-κB (thiazolidinediones) or JAK/STAT (baricitinib) in the inflammatory pathway (Toth-manikowski and Atta 2015).
Metabolomics and CKD/DKD
Recently, a new approach has been taken to study DKD. Metabolomics is being used to help understand DKD and CKD at a biochemical level. The need for a biochemical understanding of the disease stems from the lack of sensitivity and specificity of current biomarkers. For example, high urinary albumin is considered a hallmark of DKD, but is not always seen in patients with diabetes-related renal impairment. In addition, the lack of specific and omnipresent biomarkers creates a clinical situation in which DKD is not diagnosed until the more advanced stages of the disease when significant loss of GFR has already occurred. Metabolomics is a system-based approach to profile the metabolic status of a patient in vivo to aid in the identification of disease biomarkers. It is a comprehensive way to identify and quantify the end products of metabolic processes using small amounts of readily available clinic samples, like urine (Darshi et al. 2016). Metabolomics can be done on blood plasma or urine, which show different metabolic patterns. Plasma metabolomics samples circulating metabolites, while urinary metabolomics samples metabolic end products excreted by the kidney (Solini et al. 2016). The application of metabolomics to human diseases is rapidly expanding. It is expected that this new technology will provide scientists with a new view of some old and established disease processes, like DKD.
Recently, a unique signature has been characterized for DKD using metabolomics (Darshi et al. 2016). Changes in the urinary metabolites observed in DKD patients likely arise from changes in both systemic metabolism and local kidney damage typical of the diabetic state. DKD is linked to mitochondrial dysfunction, such as reduced mitochondrial biogenesis and function, which gives rise to an array of metabolic changes including organic acids, TCA cycle, urea cycle, lipid metabolism, and amino acid metabolism (Posada-Ayala et al. 2014; Darshi et al. 2016). Typical DKD-associated biomarkers include creatinine, aspartic acid, citrulline, and kynurenine, which increase and azelaic acid and galactaric acid which decrease with disease (Hirayama et al. 2012). Some metabolites may reflect a decline in kidney function (Ng et al. 2012). These authors found 11 metabolites which have a strong association with estimated glomerular filtration rate (eGFR). The strongest predictors of functional decline were octanol, oxalic acid, phosphoric acid, benzamide, creatine, 3/5 dimethoxymendelicamide, and N-Acetyl glutamine. Altered tryptophan metabolism, including acyl carnitines, has also been identified as predictors of progression of DKD and albuminuria (van der Kloet et al. 2012). Finally, a recent study identified seven metabolites as a urinary metabolomic signature of DKD (Posada-Ayala et al. 2014). These metabolites included an increase in glutamate, guanidinoacetate, alpha-phenylacetylglutamine, and trimethylamine N-oxide and a decrease in 5-oxoproline, taurine, and citrate. These urinary metabolic changes associated with DKD provide investigators with new clues into the mechanism of disease and provide a non-invasive biomarker library to guide future clinical studies.
HBOT current clinical practice
Hyperbaric oxygen therapy (HBOT) is a therapy currently used for the treatment of 14 diagnoses selected by the Undersea and Hyperbaric Medicine Society (UHMS) and the Center for Medicare and Medicaid Services (CMS) including carbon monoxide poisoning, air and gas embolisms, diabetic foot ulcers, radiation tissue injury, and other chronic wounds (Gill and Bell 2004; Godman et al. 2010). HBOT is 100% oxygen administered inside a rigid hyperbaric chamber at pressures above 1 atm (760 mmHg) (Al-Waili and Butler 2006). HBOT increases the level of oxygen in tissues by complete saturation of hemoglobin and increasing the partial pressure of oxygen dissolved in plasma. This enables oxygen to diffuse into tissues compromised by acute inflammation and microvascular disease and dysfunction. HBOT when administered at 2.0 atm will increase the total amount of oxygen carried within the bloodstream by 10-fold. Oxygen dissolved in plasma will increase from 3 ml/L when breathing 21% oxygen at 1 atm pressure to 52 ml/l when breathing 100% oxygen at 2.4 atm (Gill and Bell 2004).
A result of breathing in 100% oxygen at high pressure is the increased production of reactive oxygen species, or ROS. ROS at high levels can be damaging to tissues, but at lower levels, they can act as signaling molecules in a variety of different signaling cascades for growth factors, hormones, and cytokines (Thom 2011). ROS can also stimulate a number of cellular antioxidant and proteotoxic defense systems. During the exposure of patients to HBOT, the levels of oxygen in all tissues, including hypoxic tissues, are rapidly increased (Verma et al. 2015). This leads to the activation of fibroblasts and mobilization of macrophages and bone marrow–derived vascular endothelial cells. These cellular responses further stimulated neoangiogenesis and vasculogenesis within tissues. These complex cellular and tissue responses are known to support tissue repair and wound healing within chronic ulcerations due to diabetes and radiation tissue injury (Al-Waili and Butler 2006). Finally, HBOT has been shown to synergize with many conventional drug therapies and thereby increase treatment efficacy (Thom 2011).
Inflammatory hypoxia is recognized as a common barrier to reparative responses in many chronically diseased tissues, from autoimmune inflammatory conditions to chronic wounds and many solid tumors (Eltzschig and Carmeliet 2011; Colgan et al. 2013; Perdrizet 2017). HBOT activates anti-inflammatory mechanisms by suppressing NF-kB expression and reducing levels of TNF and IL-1B. We recently demonstrated this effect in an obese rodent model of T2DM (Weisz et al. 1997; Verma et al. 2015; Meng et al. 2016; Liu et al. 2018). After exposure to HBOT, pro-inflammatory stimulus-induced cytokine production is transiently repressed, whereas cytokine release by unstimulated and lipopolysaccharide challenged macrophages is increased. IFNγ, an anti-angiogenic cytokine, is markedly decreased following HBOT. TNF levels are similarly decreased after HBOT treatment, which helps in the repair following ischemia-reperfusion injury (injury resulting from the return of blood flow to an area that previously lacked blood flow and oxygen). HBOT also works to reduce prostaglandin production, which normally induce inflammation pain, swelling, and increased sensitivity to pain. The inhibition of this PG pathway is therefore hypothesized to play a role in HBOT’s anti-inflammatory effect and ability to reduce pathologic tissue edema (Rachmilewitz et al. 1998). This anti-inflammatory-HBOT effect is comparable to treatment with corticosteroids however without the negative side effects of the later (especially immunosuppression and hyperglycemia) (Al-Waili and Butler 2006). It may seem paradoxical that HBOT reduces inflammatory signaling, while increasing ROS production. As discussed below, the key finding in solving this paradox is the ability of HBOT-generated ROS to stimulate cellular antioxidant defense systems to suppress the “spread” of inflammation in the tissue. ROS are not all alike; those generated by an anti-inflammatory response vs those associated with hyperoxia do not have equivalent physiologic effects.
HBOT also stimulates the production of new blood vessels and their supporting collagenous matrix, by stimulating angiogenesis in response to hyperoxia (Sheikh et al. 2000). HBOT increases localized angiogenic stimuli to mobilize, recruit, and differentiate bone marrow–derived circulating stem and endothelial cells, which promote tissue neoangiogenesis and vasculogenesis. One mechanism by which HBOT induces neoangiogenesis is through induction of nitric oxide synthesis within the bone marrow, leading to the mobilization of CD34+ endothelial progenitor cells (EPC) (Thom et al. 2006). EPC and other stem cells are shown to home to ischemic tissues by a SP-1-dependent mechanism. Once established, progenitor cells differentiate and recruit other reparative cell types through release of soluble mediators locally. Paradoxically, HBOT elevates hypoxia inducible factor in EPCs, where they then act to stimulate genes, such as VEGF, involved in neovascularization (Sheikh et al. 2000; Thom 2011).
Theory supporting HBOT use for DKD
HBOT has been used to successfully treat diabetics with severe foot ulcers (Wagner Grades 3 and 4). Improved healing results in limb salvage. Randomized controlled clinical trials have shown a statistically significant reduction in major amputation rates in the setting of DFU (Löndahl 2013). It also improves DFU healing, with an odds ratio of 11.6:1 (Thom 2011). If the underling pathophysiology of DM complications is due to vascular disease, then DFU and DKD could be considered tissue-specific manifestations of the same underlying pathology. If HBOT is able to restore vascular health to the lower extremity and result in an effective tissue-reparative response, might not this response also provide benefit to the kidney developing DKD? We have demonstrated in an obese rodent model of T2DM that HBOT can reduce Caspase 3 activity in urine samples compared to sham-treated animals. We observe that therapeutic HBOT exposure activates the cytoprotective pathways within the renal tissue which, in conjunction with the known anti-inflammatory and cytoproliferative effects may affect renal repair (Verma et al. 2015). The concept of HBOT-related protection and repair of renal tissues is supported by our novel finding that HBOT upregulates NRF2-dependent antioxidant pathways with in tissues of the obese T2DM mouse. Furthermore, the family of molecular chaperones (HSPs and hemoxygenase-1) which plays critical roles in protein folding and attenuation of oxidative damage in tissues was also activated by HBOT (Rothfuss et al. 2001; Godman et al. 2010). These protective antioxidant genes may be repressed due to tissue hypoxia within or adjacent to the site of tissue damage and inflammation. The problem of inflammatory hypoxia that is known to drive many chronic inflammatory diseases is likely an important component in the complex pathophysiology of DKD (Fu et al. 2016). Furthermore, the renal tissue, due to the need to preserve very high osmolar concentration gradients via a process of counter-current exchange may be uniquely vulnerable to tissue hypoxia, especially medullary hypoxia. We suggest that oxygen is the only drug that is capable of directly reversing pathologic hypoxia within the renal medullary tissues.
In 2015, Verma et al. reported a study using db/db mice (a leptin deficient, obese, T2DM mouse model), that tested the hypothesis that HBOT may reduce DM-associated renal damage. A variety of different urinary biomarkers (NAC, NGAL, KIM1, CyC) were tested in this study, which demonstrated that 20 weeks of HBOT provided a cytoprotective effect within renal tissue. In particular, HBOT reduced the urinary levels of CyC, the biomarker most closely related to tubule functioning and an indicator of glomerular filtration rate. This is indicative of suppression of tissue damage in both glomeruli and proximal convoluted tubules and resultant maintenance of kidney functioning. In addition, neutrophil gelatinase-associated lipocalin (NGAL), a renal marker of injury from ischemia and inflammation, was found to be a particularly sensitive responder to HBOT. NGAL levels decreased following HBOT suggesting a repair of early damage associated with DKD in this mouse model (Verma et al. 2015). HBOT at 2.4 ATA significantly decreased the albumin to creatinine ratio db/db mice compared to untreated controls and is consistent with reduced glomerular membrane damage. These results were considered to be consistent with protection of the microvascular glomerular filtration membrane, even though GFR itself was not directly studied.
Increased glucose concentration in the db/db mice leads to the generation of excess ROS from mitochondria, ER, and oxygen-utilizing enzymes of the NADPH oxidase family. These ROS seem to underlie the creation of chronic inflammation that can lead to end-stage renal failure (ESRD). It is interesting to note that hypoxia and HBOT both increase ROS, which underlies some of the pathogenesis of DKD and progression to ESRD. Verma et al. 2015 address this paradox by suggesting that HBOT delivers the oxygen-saturated blood into tissues, restoring normal oxygen levels, thereby reducing endogenous, pathologic ROS production. This may happen by preventing the excessive loading of electron carriers in the electron transport chain in mitochondria. The electron carriers become overloaded in hypoxic states, and transfer extra electrons to oxygen when it periodically appears. These then become the toxic ROS (Nathan 2008). HBOT in turn works to reduce the production of endogenous ROS by restoring normal oxygen levels within tissues and decreasing the load on electron carriers through tissue (mitochondrial) oxygenation, thereby reducing the amount of pathologic ROS (Verma et al. 2015; Zhou et al. 2018). HBOT enables oxygen to penetrate compromised and diseased tissues and thereby improves mitochondrial function and with it restores tissue bioenergetics to support healing (Zhou et al. 2018).
There are many differences between diabetic foot ulceration and diabetic kidney disease. We do wish to re-emphasize that the hyperglycemia of diabetics has a systemic effect which causes widespread vascular disease, both macrovascular and microvascular, and it is this underlying vasculopathy that is the fundamental basis for the organ and tissue-specific manifestations of the diabetic complications—retinopathy, nephropathy, neuropathy, cardiovascular disease, and cerebrovascular disease. This fundamental diabetic vasculopathy is also the specific target of HBOT, a systemic treatment. Large, well-powered randomized controlled trials are needed to determine the ultimate efficacy and real-world effectiveness of this treatment. Unfortunately, wounds may not heal and amputations may be performed and neither may be the direct result of a failure in the biologic healing response. The complex nature of this problem underlies the ongoing controversy about the effect of HBOT on clinical wound healing rates and lower extremity amputation rates (Santema et al. 2018; Margolis et al. 2013; and related editorials).
Current study data
A prospective study of 35 diabetic patients receiving HBOT for the treatment of a DFU was designed to determine if 30 HBOT sessions (2.4 atm, 90 min per session, daily 4–5 days/week) could alter the urinary metabolomic profile of these unselected patients by comparing pretreatment to post-treatment urinary samples. During the study, all patients received the standard of care for their diabetes and foot ulcers. The working hypothesis was that HBOT would improve metabolic markers related to oxidant and inflammatory tissue injury, especially those known be altered in DKD, namely mitochondrial metabolites. A secondary hypothesis was that improvement in metabolomic profiles would be associated with reduced albuminuria as measured by the urinary albumin: creatine ratio. This preliminary report presents data from the initial 17 patients enrolled in this IRB committee approved study. After providing written informed consent for research, patients were treated with daily HBOT sessions, 4–5 days per week for an average of 30 total sessions. During each session, patients breathed 100% oxygen at 2.4 atm within a multiple-person hyperbaric chamber for 90 min per session. Patient samples were collected before the first and after the 25–30th treatment session. All collected samples were immediately placed on ice and then frozen at − 80 °C within 2 h. All samples underwent preliminary point of care testing to rule out acute urinary tract infection or hemorrhage. One sample tested positive for infection and was excluded from this study. Samples were then transported on dry ice over night to Metabolon Corp., Research Triangle Park, NC for analysis of 778 compounds of known identities and then performed proprietary analysis.
Metabolon Corp. prepared the samples using the MicroLab STAR system from Hamilton Company. Several controls were implemented in the analysis such as a pooled mixture of all samples, water blanks, and QC standards. Ultrahigh performance liquid chromatography and tandem mass spectroscopy were performed on each sample. Bioinformatics was performed using Laboratory Information Management System (LIMS) so that there could be fully auditable laboratory automation. Data could then be extracted and compounds could be identified through comparison to library entries from purified standards.
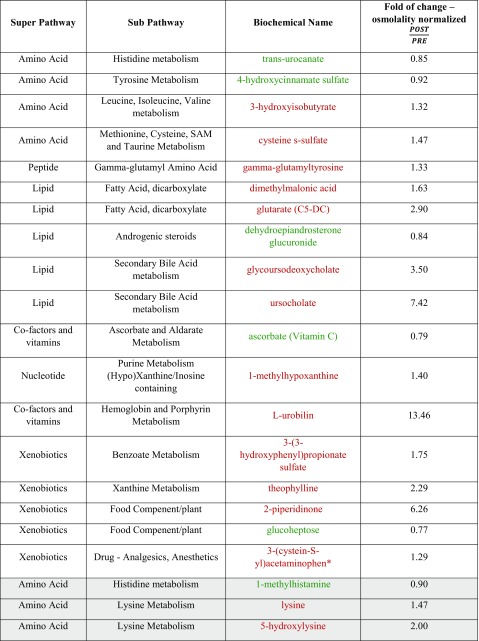
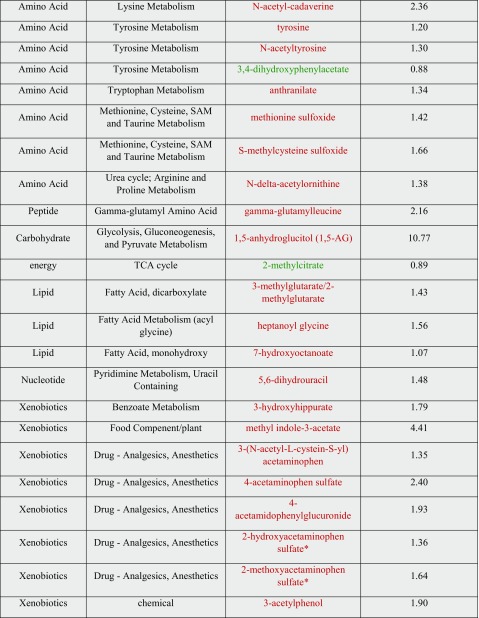
Of the 778 metabolites, 18 were identified that significantly changed after HBOT exposure (p ≤ 0.05), with 13 increasing and 5 decreasing. Another 26 metabolites were identified for their trending toward significance (0.05 < p < 0.10), with 23 increasing and 3 decreasing. Of the metabolites showing significant changes after HBOT exposure, many that increased after treatment were lower in the diabetic urine before treatment when compared to the control urine. These results are displayed in Table 1.
Table 1.
List of metabolites showing statistical difference after HBOT treatment
White background represents p < 0.05, gray is 0.05 < p < 0.10. Red text represents an increase in change and green shows a decrease
After HBOT treatment was completed, there was an increase in the oxidative stress marker methionine sulfoxide (1.42, 0.05 < p < 0.10) and an increase in l-urobilin (13.46, p ≤ 0.05), which is a heme degradation product. The combination of these two metabolites being present in the urine after treatment is consistent with the induction of oxidative stress. Although an increase in ROS is suggested to play a role in the pathogenesis of DKD, it has been noted that physiologic levels of ROS can have a positive effect on the body, especially in relation to cell signaling and the activation of protective stress-response genes through Nrf2 and HSF (Coughlan and Sharma 2016). Physiological ROS signaling is seen as beneficial whereas pathological ROS regaling can be damaging. ROS can act as second messengers to aid in the homeostasis of cellular function through regulation of important pathways (Coughlan and Sharma 2016). The increase in ROS seen here is a good parallel to the 2015 Verma study where it was hypothesized that HBOT leads to better delivery of oxygen-saturated blood to tissues in order to normalize oxygen levels, thereby decreasing production of endogenous ROS. They proposed that HBOT produced low levels of ROS that stimulated cellular antioxidant defenses, which suppressed excessive ROS generation associated with the inflammatory response. Additionally, HBOT increases cytoprotection through decreasing expression of the HSPA1A (heat shock protein), preventing protein denaturation (Verma et al. 2015). The concomitant decrease in vitamin C metabolites, such as ascorbate (0.79, p < 0.05), following HBOT suggests that there is an increase in the detoxification of oxygen radicals or potentially better tubular reabsorption of vitamin C. This observation is consistent with increased oxidative signaling and antioxidant defenses arising from higher tissue oxygen tensions after HBOT exposure (Rothfuss et al. 2001; Bader et al. 2007) (Fig. 1).
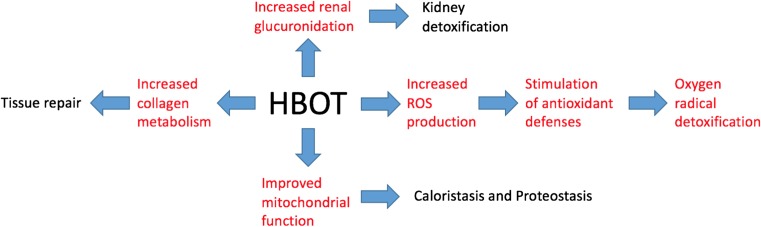
Fig. 1.
Cellular and tissue effects of HBOT related to kidney protection. Effects elucidated through the metabolomic analysis described here are shown in red
Many small organic acids were increased in concentration following HBOT treatment. These included dimethylmalonic acid (1.63, p < 0.05), glutarate (2.90, p < 0.05), 3-methylglutarate/2-methylglutarate (1.43, 0.05 < p < 0.10), and 3-hydroxisobuyrate (1.32, p < 0.05). Glutarate, 3-methylglutarate/2-methylglutarate and 3-hydroxybutyrate are all known products of mitochondrial protein catabolism and fatty acid catabolism, processes which take place in the mitochondria (Sharma et al. 2013). In previous metabolomic studies involving diabetic patients, metabolites from the citric acid cycle, pyrimidine metabolism, amino acid metabolism, and fatty acid are all decreased in diabetic patients with DKD compared to diabetic controls without renal damage. Many of these metabolites are produced in the mitochondria, which suggests that there is a decline in the functioning of the mitochondria in patients with DKD (Coughlan and Sharma 2016). The relative increase in these small organic metabolites may be indicative of better mitochondrial functioning after HBOT treatment.
Changes in mitochondrial function have major consequences for the cell’s ability to maintain cellular energy homeostasis. As more metabolomic data begins to emerge through further studies such as those mentioned above and the data presented later in this paper, it may be useful to compile a “caloristasis network” (Bonorino et al. 2018). There is a critical link between proteostasis and cellular energy homeostasis, as protein metabolism is the most energy-expensive process in most cells (Lindqvist et al. 2018). This term “caloristasis network” can be used to encompass the metabolic pathways and processes that contribute to the production, maintenance, and use of cellular energy. This network will echo and overlap the widely studied proteostasis network.
Glucuronidation is the process responsible for pharmacological drug metabolism, relying on a series of oxidation and conjugation reactions (Hoyumpa and Schenker 1991). This process occurs primarily in the liver, but also takes place in the kidneys (Vree et al. 1992). The data show that many glucuronidated molecules are elevated post-HBOT exposure, including 4-acetaminophenylglucuronide (1.93, 0.05 < p < 0.10) and a few partially characterized glucuronidated molecules. This is indicative of potential increases in renal glucuronidation or increased renal clearance of glucuronidated products. Changes in glucuronidation may play a role in the development of DKD, but further studies are needed to confirm whether or not HBOT increases glucuronidation (Zhao et al. 2012).
Two products of secondary bile acid metabolism, uroscholate (7.42, p < 0.05) and glycoursodeoxycholate (3.50, p < 0.05), increase after HBOT. Bile acids are typically absorbed and excreted by the liver, but can occasionally escape hepatic reabsorption. If this is the case, bile acids can be filtered by the kidney and reabsorbed in the proximal tubule. The fact that two bile acid products are increased in the urine after HBOT may indicate changes in bile acid synthesis, microbiome changes in the gastrointestinal tract, a decrease in hepato-portal bile acid reuptake, increased renal clearance, or a decrease in the renal reabsorption (Ballatori et al. 2005). There is evidence that bile salts might be toxic to the renal tubules (Fickert et al. 2013). However, only these two bile acids significantly changed (p < 0.10) out of the 11 secondary bile acid metabolites and 7 primary bile acid metabolites, making it difficult to say what implication this finding has on understanding HBOT effects.
The metabolomic data also suggest that HBOT may lead to an altered collagen metabolism, with the type I collagen/remodeling metabolite 5-hydroxylysine (2.00, 0.05 < p < 0.10) showing an increased level in urine post-treatment (Yamauchi and Sricholpech 2012). Type IV collagen is linked to a decline in kidney function as increases in type IV collagen are highly correlated with albuminuria. Type I collagen however has not been closely linked to DKD, leading to the puzzling question of how type I collagen fits into the DKD picture (Jha et al. 2016). Of note, HBOT increases tissue repair and collagen deposition in the context of wound healing (André-Lévigne et al. 2017), so this finding may be reflective of a systemic increase in tissue remodeling. Still, not all collagen metabolites changed, again suggesting further study is necessary to better understand the complex relationship of collagen synthesis to DKD and HBOT. In addition, 5-hydroxylysine is a vitamin C–dependent product. The finding of increased 5-hydroxylysine is interesting in light of a concomitant reduction in vitamin C levels associated with HBOT. This may be an indication that more ascorbate (vitamin C) is used in producing collagen, thereby reducing ascorbate concentrations but increasing collagen metabolites (Murad et al. 1981).
Conclusion
After 1 month of daily HBOT treatments for DFU, this preliminary metabolomic analysis shows that significant changes are present within the T2DM patient compared to healthy, non-diabetic control and that these changes responded to HBOT. Other biochemical effects of HBOT identified by this report include increased antioxidant defenses, increased renal (the liver does this, I believe) glucuronidation, changes in bile acid levels, and altered collagen synthesis. HBOT resulted in an increase in mitochondrial metabolites and suggests an increase in mitochondrial function. The current study is in the final stages of patient recruitment with a goal to obtain a total n = 35. At this point, it is reasonable to state that HBOT, in the setting of DFU, has both a systemic and pleiotropic effect on an array of metabolites. Correlation with organ function and clinical outcomes is needed.
References
- Altemtam N, Nahas ME, Johnson T. Urinary matrix metalloproteinase activity in diabetic kidney disease: a potential marker of disease progression. Nephron Extra. 2012;2:219–232. doi: 10.1159/000339645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waili NS, Butler GJ. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. Sci World J. 2006;6:425–441. doi: 10.1100/tsw.2006.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André-Lévigne Dominik, Modarressi Ali, Pepper Michael, Pittet-Cuénod Brigitte. Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair. International Journal of Molecular Sciences. 2017;18(10):2149. doi: 10.3390/ijms18102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader N, Bosy-Westphal A, Koch A, Rimbach G, Weimann A, Poulsen HE, Müller MJ. Effect of hyperbaric oxygen and vitamin C and E supplementation on biomarkers of oxidative stress in healthy men. Br J Nutr. 2007;98:826–833. doi: 10.1017/S0007114507744380. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTα-OSTβ: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Bonorino C, Sistonen L, Eriksson J, Mezger V, Santoro G, Hightower LE. The VIII international congress on stress proteins in biology and medicine: taynna henkea. Cell Stress Chaperones. 2018;23:171–177. doi: 10.1007/s12192-018-0878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Curtis VF, Campbell EL. The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis. 2013;19:2238–2244. doi: 10.1097/MIB.0b013e31828dcaaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan MT, Sharma K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 2016;90:272–279. doi: 10.1016/j.kint.2016.02.043. [DOI] [PubMed] [Google Scholar]
- Darshi M, Van Espen B, Sharma K. Metabolomics in diabetic kidney disease: unraveling the biochemistry of a silent killer. Am J Nephrol. 2016;44:92–103. doi: 10.1159/000447954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Krones E, Pollheimer MJ, Thueringer A, Moustafa T, Silbert D, Halilbasic E, Yang M, Jaeschke H, Stokman G, Wells RG, Eller K, Rosenkranz AR, Eggertsen G, Wagner CA, Langner C, Denk H, Trauner M. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology. 2013;58:2056–2069. doi: 10.1002/hep.26599. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A. Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia. 2000;43:1205–1223. doi: 10.1007/s001250051515. [DOI] [PubMed] [Google Scholar]
- Fu Q, Colgan SP, Shelley CS. Hypoxia: the force that drives chronic kidney disease. Clin Med Res. 2016;14:15–39. doi: 10.3121/cmr.2015.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H, Suckling RJ. Diabetic nephropathy: where are we on the journey from pathophysiology to treatment? Diabetes Obes Metab. 2016;18:641–647. doi: 10.1111/dom.12630. [DOI] [PubMed] [Google Scholar]
- Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM - Mon J Assoc Physicians. 2004;97:385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin DG, Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010;15:431–442. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama A, Nakashima E, Sugimoto M, Akiyama SI, Sato W, Maruyama S, Matsuo S, Tomita M, Yuzawa Y, Soga T. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem. 2012;404:3101–3109. doi: 10.1007/s00216-012-6412-x. [DOI] [PubMed] [Google Scholar]
- Hoyumpa AM, Schenker S. Is glucuronidation truly preserved in patients with liver disease? Hepatology. 1991;13:786–795. doi: 10.1002/hep.1840130428. [DOI] [PubMed] [Google Scholar]
- Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Islam S, Rouf A, Raghav A, et al. Neo-epitopes generated on hydroxyl radical modified glycatedigg have role in immunopathology of diabetes type 2. PLoS One. 2017;12:e0169099. doi: 10.1371/journal.pone.0169099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha JC, Banal MC, Chow BSM, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S (2013) Diabesity. J Pak Med Assoc
- Lindqvist LM, Tandoc K, Topisirovic I, Furic L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr Opin Genet Dev. 2018;48:104–111. doi: 10.1016/j.gde.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Su, Lu Chun, Liu Ying, Zhou Xiaoyun, Sun Li, Gu Qi, Shen Guangyu, Guo Aisong. Hyperbaric Oxygen Alleviates the Inflammatory Response Induced by LPS Through Inhibition of NF-κB/MAPKs-CCL2/CXCL1 Signaling Pathway in Cultured Astrocytes. Inflammation. 2018;41(6):2003–2011. doi: 10.1007/s10753-018-0843-2. [DOI] [PubMed] [Google Scholar]
- Löndahl M. Hyperbaric oxygen therapy as adjunctive treatment of diabetic foot ulcers. Med Clin North Am. 2013;97:957–980. doi: 10.1016/j.mcna.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M. Lack of effectiveness of hyperbaric diabetic foot ulcer and the prevention of amputation. Diabetes Care. 2013;36:1961–1966. doi: 10.2337/dc12-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X-E, Zhang Y, Li N, Fan DF, Yang C, Li H, Guo DZ, Pan SY. Hyperbaric oxygen alleviates secondary brain injury after trauma through inhibition of TLR4/NF-κB signaling pathway. Med Sci Monit. 2016;22:284–288. doi: 10.12659/MSM.894148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78:2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14:485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- Ng D. P. K., Salim A., Liu Y., Zou L., Xu F. G., Huang S., Leong H., Ong C. N. A metabolomic study of low estimated GFR in non-proteinuric type 2 diabetes mellitus. Diabetologia. 2011;55(2):499–508. doi: 10.1007/s00125-011-2339-6. [DOI] [PubMed] [Google Scholar]
- Perdrizet G. A. Advances in Experimental Medicine and Biology. Cham: Springer International Publishing; 2017. Chronic Diseases as Barriers to Oxygen Delivery: A Unifying Hypothesis of Tissue Reoxygenation Therapy; pp. 15–20. [DOI] [PubMed] [Google Scholar]
- Posada-Ayala M, Zubiri I, Martin-Lorenzo M, Sanz-Maroto A, Molero D, Gonzalez-Calero L, Fernandez-Fernandez B, de la Cuesta F, Laborde CM, Barderas MG, Ortiz A, Vivanco F, Alvarez-Llamas G. Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int. 2014;85:103–111. doi: 10.1038/ki.2013.328. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Karmeli F, Okon E, Rubenstein I, Better OS. Hyperbaric oxygen: a novel modality to ameliorate experimental colitis. Gut. 1998;43:512–518. doi: 10.1136/gut.43.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfuss A, Radermacher P, Speit G. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogenesis. 2001;22:1979–1985. doi: 10.1093/carcin/22.12.1979. [DOI] [PubMed] [Google Scholar]
- Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower-extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care. 2018;41(1):112–119. doi: 10.2337/dc17-0654. [DOI] [PubMed] [Google Scholar]
- Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh a Y, Gibson JJ, Rollins MD, et al. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg. 2000;135:1293–1297. doi: 10.1001/archsurg.135.11.1293. [DOI] [PubMed] [Google Scholar]
- Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J Clin Endocrinol Metab. 2016;101:696–704. doi: 10.1210/jc.2015-3345. [DOI] [PubMed] [Google Scholar]
- Thom SR. Hyperbaric oxygen-its mechanism and efficacy. Plast Reconstr Surg. 2011;127:1–16. doi: 10.1097/PRS.0b013e3181fbe2bf.Hyperbaric. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–H1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- Toth-Manikowski Stephanie, Atta Mohamed G. Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets. Journal of Diabetes Research. 2015;2015:1–16. doi: 10.1155/2015/697010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Renal Data System. (2017). 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. 10.1053/j.ajkd.2017.01.020 [DOI] [PMC free article] [PubMed]
- van der Kloet FM, Tempels FWA, Ismail N, van der Heijden R, Kasper PT, Rojas-Cherto M, van Doorn R, Spijksma G, Koek M, van der Greef J, Mäkinen VP, Forsblom C, Holthöfer H, Groop PH, Reijmers TH, Hankemeier T. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study) Metabolomics. 2012;8:109–119. doi: 10.1007/s11306-011-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Chopra A, Giardina C, Sabbisetti V, Smyth JA, Hightower LE, Perdrizet GA. Hyperbaric oxygen therapy (HBOT) suppresses biomarkers of cell stress and kidney injury in diabetic mice. Cell Stress Chaperones. 2015;20:495–505. doi: 10.1007/s12192-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vree TB, Hekster YA, Anderson PG. Contribution of the human kidney to the metabolic clearance of drugs. Ann Pharmacother. 1992;26:1421–1428. doi: 10.1177/106002809202601116. [DOI] [PubMed] [Google Scholar]
- Weisz G, Lavy A, Adir Y, Melamed Y, Rubin D, Eidelman S, Pollack S. Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J Clin Immunol. 1997;17:154–159. doi: 10.1023/A:1027378532003. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113–133. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang H, Zhao T, Zhang X, Lu J, Yin T, Liang Q, Wang Y, Luo G, Lan H, Li P. Intrarenal metabolomics reveals the association of local organic toxins with the progression of diabetic kidney disease. J Pharm Biomed Anal. 2012;60:32–43. doi: 10.1016/j.jpba.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Huang G, Yu X, Weigang X. A novel approach to estimate ROS origination by hyperbaric oxygen exposure, targeted probes and specific inhibitors. Cell Physiol Biochem. 2018;47:1800–1808. doi: 10.1159/000491061. [DOI] [PubMed] [Google Scholar]