Figure 6.
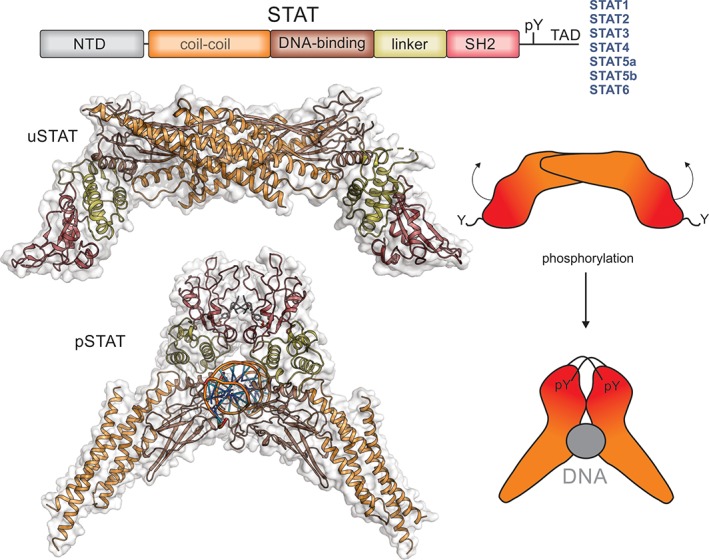
STATs. The Signal Tranducers and Activators of Transcription (STATs) are a family of latent transcription factors that are activated by phosphorylation following cytokine exposure. The same domain architecture is shared by all STAT proteins and is shown schematically above. Unphosphorylated STAT (uSTAT) exists as an antiparallel dimer in the cytoplasm (upper). The SH2 domain (red) of uSTAT binds to phosphotyrosines in cytokine receptors which allows JAK to phosphorylate a specific tyrosine located between the SH2 and transactivation domain (TAD). This phosphotyrosine is then targeted by the SH2 domain of the other monomer inducing a large rotation between the two subunits of the dimer and allowing phosphorylated STAT (pSTAT) to occupy its DNA‐binding competent dimeric structure (lower). The structures shown here are of STAT1 (PDB ID: http://firstglance.jmol.org/fg.htm?mol=1YVL,135 http://firstglance.jmol.org/fg.htm?mol=1BF5 136) with the colors matching the schematic representation above. The N‐terminal domain of STAT does not appear to form a stable interaction with the rest of the molecule and is not shown here. The transactivation domain (TAD) is unstructured but allows binding of accessory factors.
