Figure 8.
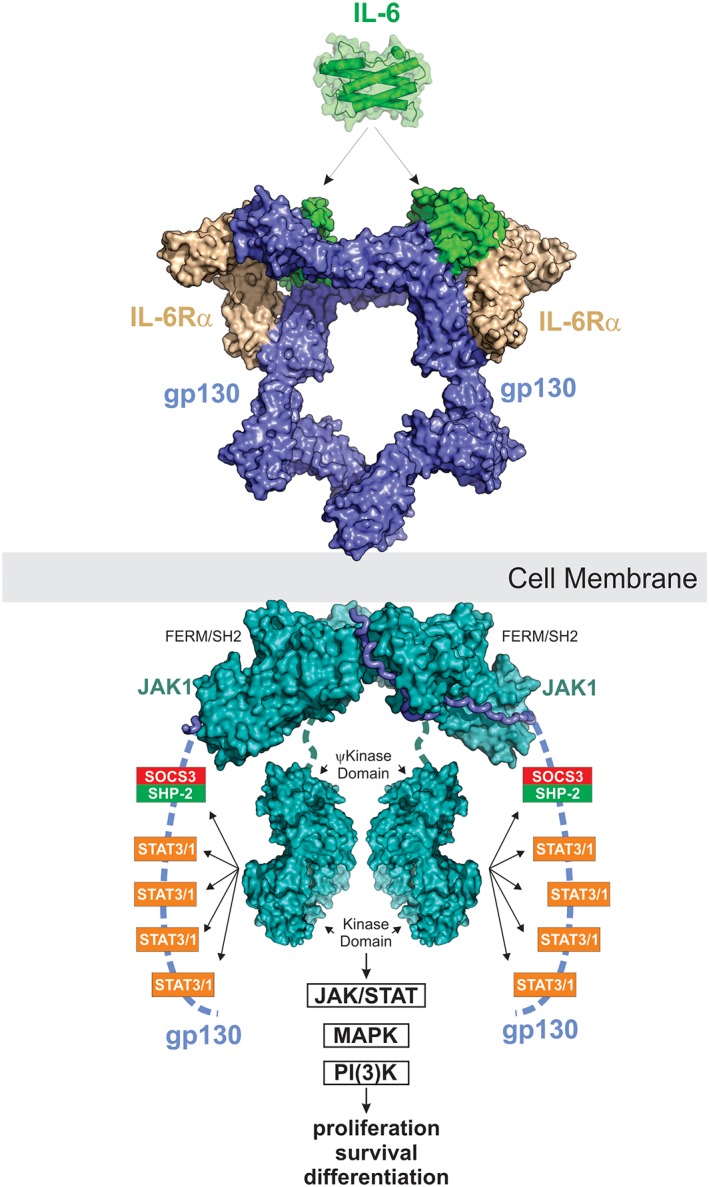
IL‐6 signaling. IL‐6 signals via a 2:2:2 complex between itself, gp130 and either membrane‐bound IL‐6Rα (classic signaling) or soluble IL‐6Rα (trans‐signaling). JAK1, JAK2 and TYK2 can all bind the intracellular domain of gp130; however, JAK1 appears to be the dominant kinase. The structure of JAK1 bound to the gp130 cytoplasmic domain is a model based on the structures of JAK1/IFNλR (PDB ID: http://firstglance.jmol.org/fg.htm?mol=5L04) and the JAK2/EPOR dimeric structure (coordinates kindly provided by R. Ferrao and P. Lupardus). JAK is activated by trans‐phosphorylation and then phosphorylates five tyrosine residues on the receptor intracellular domain. The four distal tyrosines are docking sites for STAT3 and to a lesser degree STAT1. Activated STAT3 is then phosphorylated by JAK and translocates into the nucleus to drive the biological response. The MAPK pathway is also stimulated by IL‐6 via SHP2 which binds to pY759 using its SH2 domain. The PI(3)K pathway is also activated in response to IL‐6. SOCS3 is a direct STAT3 target gene and binds to the SHP2‐binding site on the receptor via its SH2 domain. This inhibits MAPK signaling via displacement of SHP2 and also inhibits further STAT3 activation by direct inhibition of JAK catalytic activity.
