Abstract
We report the case of a 42-year-old patient referred to our department for the examination of two large, symmetrical inguinal lumps. The ultrasound examination of the swollen lymph node demonstrated a cortical echogenicity greater than the medullary echogenicity, and the vascularization stop around the cortical zone suggested a pathological pattern of mantle cell lymphoma. In this type of lymphoma, lymphocytes are localized in a mantle zone, inducing a thickening of the lumps. Therefore, for the first time, ultrasound examination detected sonographic vascular features of mantle cell lymphoma, allowing the identification of the disease and suggesting the specific histological diagnosis.
Keywords: Mantle cell lymphoma, Lymph nodes, Lymph node mantle, Ultrasound
Sommario
Riportiamo il caso di una paziente di 42 anni giunta alla nostra osservazione per il riscontro di due grossi linfonodi inguinali simmetrici. L’esame ecografico del linfonodo ingrossato ha mostrato una ecogenicità corticale maggiore di quella midollare, e il blocco della vascolarizzazione attorno alla zona corticale ha evidenziato un pattern patologico suggestivo di linfoma a cellule mantellari. In questo tipo di linfoma, i linfociti sono localizzati in una zona del mantello, inducendo un ispessimento dei linfonodi. Pertanto, per la prima volta in letteratura, l’esame ecografico ha rilevato delle caratteristiche vascolari ecografiche di linfoma a cellule mantellari, consentendo l’identificazione della malattia e suggerendo la diagnosi istologica specifica.
Introduction
Mantle cell lymphoma (MCL) is a mature B-cell non-Hodgkin lymphoma (NHL), representing about 6–8% of adult NHL [1, 2]. It primarily affects middle-aged adults, with an average age of 65 years (male/female ratio of 4:1) [1, 3]. MCL is usually a moderately aggressive disease that often occurs in an asymptomatic manner. Patients generally present with stage III/IV disease and extensive lymphadenopathy, blood and bone marrow involvement, and splenomegaly [4]. Approximately 75% of patients display lymphadenopathy, while the remaining patients are characterized by extranodal involvement. Pancytopenia or a leukemic presentation with extensive leukocytosis may occur [5]. The involvement of the spleen and bone marrow, and a localization in the gastrointestinal tract are also common. MCL is often associated with systemic B symptoms, such as night sweats, fatigue, anorexia, and unintentional weight loss. Lymphoid cells express B-cell antigens and high levels of surface immunoglobulins [6]. MCL is often characterized by translocation t(11;14) engaging the BCL-1/Prad-1 gene, which positioned the cyclin D1 gene in close proximity to the gene of the immunoglobulin heavy chain [2, 6]. This aberration has been found in 95% of MCL cases [2]. In a small proportion of patients, MCL may arise from overexpression of other cyclin genes (for example, cyclin D2 and cyclin D3) [2]. The proliferation of neoplastic cells is associated with different growth stages: first the lymphocytes are assembled at the level of the mantle, causing a thickening of the mantle, then, infiltration deals with the follicles, until the transformation to a spread form where the architecture of the lymph nodes is deleted [2]. In the same patient, lymph nodes may be present with different infiltrative features.
Staging should include the following assessments: a complete blood count, chemistry profile, measurement of lactic acid dehydrogenase, bone marrow evaluation with immunophenotyping by flow cytometry of the bone marrow and blood, an ultrasound (US) and computed tomography (CT) of the chest, abdomen, and pelvis or a fluorodeoxyglucose positron emission tomography (FDG-PET)/CT [2, 3]. In cases presenting intestinal involvement (lymphomatous polyposis), endoscopy must be performed [7].
Case description
A 42-year-old patient came to our observation at the Internistic Ultrasound Department for the recent detection of two large, symmetrical lumps on bilateral inguinal areas. The patient reported an increase in weight, about 10 kg in 6 months (body mass index = 33), and thought that the swelling was a result of fat accumulation. The patient did not have a particular history of remote pathologies and did not take any drugs.
The patient exhibited non-specific symptoms, such as mild asthenia, and did not report fever, night sweats, or itching. Upon physical examination, the two lumps appeared to be large, oval bumps in the right and left inguinal sites. They had well-defined edges, hard consistencies, and minimal movement on the deep planes, and they were not painful. Furthermore, similar but smaller lumps were spotted in nearby areas. The liver and spleen were not palpable during abdominal physical examination. Lymphadenopathy was not detected in the armpits or in the laterocervical area.
The US examination, performed by linear and convex multi-frequency probes, showed multiple solid lumps on both sides of the inguinal region. The lumps were characterized by an oval morphology, and some of the lumps tended to merge, displaying a non-homogeneous echostructure. Moreover, different echogenicities were observed as the enlarged lymph nodes showed a peripheral increase in echogenicity compared to the central perihilar area (Fig. 1a, b).
Fig. 1.
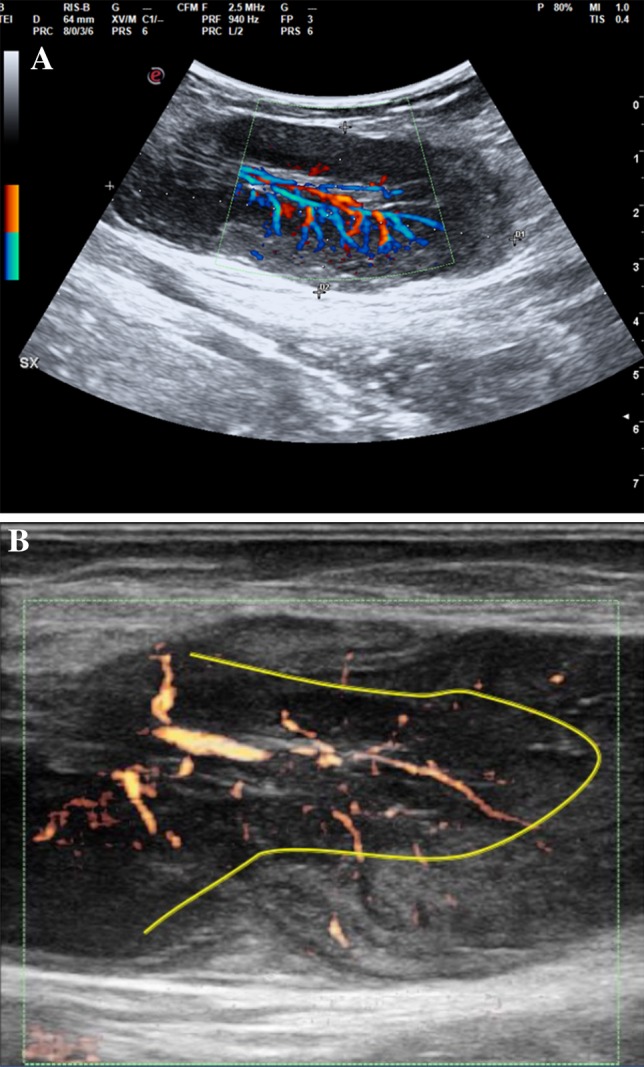
Pathological lymph node displaying hypertrophic hilar vessels; the perfusion stops its transition between the medullary and cortical areas, thus depriving most of the peripheral area of its physiological vascularization. a Color Doppler analysis; b power Doppler analysis
Color and power Doppler analysis showed hypertrophic hilar vessels with substantial perfusion. Notably, the perfusion seemed to interrupt its transition between the medullary and cortical areas, thus, depriving most of the peripheral area of its physiological vascularization (Fig. 2).
Fig. 2.
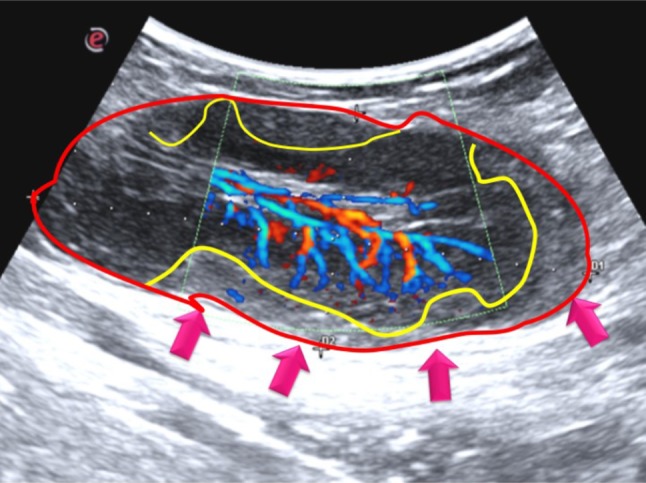
Details of pathological lymph node: it is characterized by an oval morphology, with a non-homogeneous echostructure. The different perfusion degrees between the medullary and cortical portions generate different echogenicities in nodal areas in the periphery compared to the central ones
The volume, the number of pathological lymph nodes, the short-to-long axes ratio (S/L) of the lumps, together with vascular features such as high perfusion and angioarchitectural heterogeneity, suggested a lymphoproliferative disease that resembled the features of mantle cell lymphoma. However, the case was questionable due to the detectable alterations in the peripheral area.
Histologic examination was performed subsequent to US-guided excisional biopsy of the larger inguinal lymph node, thereby confirming the sonographic suspicion of mantle cell lymphoma.
Discussion
Our case underlines the main importance of the primary use of US in the examination of palpable subcutaneous formations and in the differential diagnosis of lymph node diseases. The finding of multiple enlarged lymph nodes can be a sign of different pathologies, from inflammatory-reactive pathologies to malignant tumors. Moreover, enlarged nodes in an unexpected site may suggest neoplastic disease and that the primary tumor is biologically aggressive. In our case, the referred patient reported an unclear symptomatology that was not suggestive of a specific pathological process.
The ultrasound allowed us to confirm the lymph-nodal nature of the subcutaneous formations and to define their structural characteristics. The lymphomatous nodes tend to be round in shape, well-defined, hypoechoic, and are usually without an echogenic hilus [8]. Furthermore, pathological nodes tend to be enlarged with a minimum transverse diameter of 10 mm [8], but nodal size alone is not a specific criterion to differentiate lymphomatous nodes from normal nodes [9]. Malignant nodes tend to be round with a S/L ratio greater than 0.5, while reactive or benign lymph nodes are characterized by an elliptical shape [10]. In the present case report, the lumps were characterized by an oval morphology, and some of them tended to merge, displaying a non-homogeneous echostructure. Intriguingly, the enlarged lymph nodes displayed a peripheral increase in echogenicity compared to the central perihilar area, thus quite resembling the pathological lymphocyte accumulation in the mantle. Intranodal calcification and cystic necrosis are other less common pathological findings detected after radiation therapy or chemotherapy or in advanced disease, but none were present in our case report [11].
Power Doppler US should be performed to detect peculiar features suggestive of benign or malignant disease. Indeed, lymphomatous lymph nodes usually tend to have both hilar and peripheral vessels [12, 13]. In our case, the lymph nodes displayed different echogenicities in the peripheral areas compared to the central area. Hypertrophic hilar vessels with significant perfusion were seen, but the perfusion seemed to stop its transition between the medullary and cortical areas, thus depriving most of the peripheral area of its physiological vascularization. These sonographic findings were related to the physiopathology of the disease. Indeed, dysregulated lymphocyte proliferation leads to lymphocyte assembly at the mantle level, resulting in a thickening of the area. We found that the main sonographic and vascular changes of the lymphadenopathy were in the peripheral area as a consequence of the biological characteristics of MCL.
Conclusions
According to the current state of knowledge, the present case report is the first time that an US test (performed combining both the B-mode, and the color and power Doppler) has allowed not only the identification of a possible diagnosis of lymphoproliferative disease in a patient with poor clinical symptoms, but also for a histological diagnosis to be obtained. In the patient suspected for MCL, the B-mode and color–power Doppler US study allows not only to detect the lymphomatous enlarged lymph nodes, but also to suspect the final histological diagnosis of this subtype of lymphoma, based on different echogenicities and vascularization between the peripheral and central areas. These findings reinforce the role of US as a first-level diagnostic tool for lymph nodal disease, thus opening new intriguing opportunities to allow for a specific pre-histological lymphoma subtype diagnosis.
Funding
This study was not funded.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Zhou Y, Wang H, Fang W, Romaguer JE, Zhang Y, Delasalle KB, Kwak L, Yi Q, Du XL, Wang M. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113:791–798. doi: 10.1002/cncr.23608. [DOI] [PubMed] [Google Scholar]
- 2.Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34:1256–1269. doi: 10.1200/JCO.2015.63.5904. [DOI] [PubMed] [Google Scholar]
- 3.Vose JM. Mantle cell lymphoma: 2015 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2015;90:739–745. doi: 10.1002/ajh.24094. [DOI] [PubMed] [Google Scholar]
- 4.Tiemann M, Schrader C, Klapper W, Dreyling MH, Campo E, Norton A, Berger F, Kluin P, Ott G, Pileri S, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol. 2005;131:29–38. doi: 10.1111/j.1365-2141.2005.05716.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer A, Salaverria I, Bosch F, Villamor N, Rozman M, Bea S, Gine E, Lopez-Guillermo A, Campo E, Montserrat E. Leukemic involvement is a common feature in mantle cell lymphoma. Cancer. 2007;109:2473–2480. doi: 10.1002/cncr.22715. [DOI] [PubMed] [Google Scholar]
- 6.Aukema SM, Hoster E, Rosenwald A, Canoni D, Delfau-Larue MH, Rymkiewicz G, Thorns C, Hartmann S, Kluin-Nelemans H, Hermine O, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood. 2018;131:417–420. doi: 10.1182/blood-2017-07-797019. [DOI] [PubMed] [Google Scholar]
- 7.Romaguera JE, Medeiros LJ, Hagemeister FB, Fayad LE, Rodriguez MA, Pro B, Younes A, McLaughlin P, Goy A, Sarris AH, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003;97:586–591. doi: 10.1002/cncr.11096. [DOI] [PubMed] [Google Scholar]
- 8.Bruneton JN, Normand F, Balu-Maestro C, Kerboul P, Santini N, Thyss A, Schneider M. Lymphomatous superficial lymph nodes: US detection. Radiology. 1987;165:233–235. doi: 10.1148/radiology.165.1.3306785. [DOI] [PubMed] [Google Scholar]
- 9.van Overhagen H, Lameris JS, Berger MY, van der Voorde F, Tilanus HW, Klooswijk AI, Zonderland HM, van Pel R. Supraclavicular lymph node metastases in carcinoma of the esophagus and gastroesophageal junction: assessment with CT, US, and US-guided fine-needle aspiration biopsy. Radiology. 1991;179:155–158. doi: 10.1148/radiology.179.1.2006268. [DOI] [PubMed] [Google Scholar]
- 10.Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183:215–220. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- 11.Swartz JD, Yussen PS, Popky GL. Imaging of the neck: nodal disease. Crit Rev Diagn Imaging. 1991;31:413–469. [PubMed] [Google Scholar]
- 12.Na DG, Lim HK, Byun HS, Kim HD, Ko YH, Baek JH. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol. 1997;168:1311–1316. doi: 10.2214/ajr.168.5.9129432. [DOI] [PubMed] [Google Scholar]
- 13.Dragoni F, Cartoni C, Pescarmona E, Chiarotti F, Puopolo M, Orsi E, Pignoloni P, De Gregoris C, Mandelli F. The role of high resolution pulsed and color Doppler ultrasound in the differential diagnosis of benign and malignant lymphadenopathy: results of multivariate analysis. Cancer. 1999;85:2485–2490. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2485::AID-CNCR26>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]


