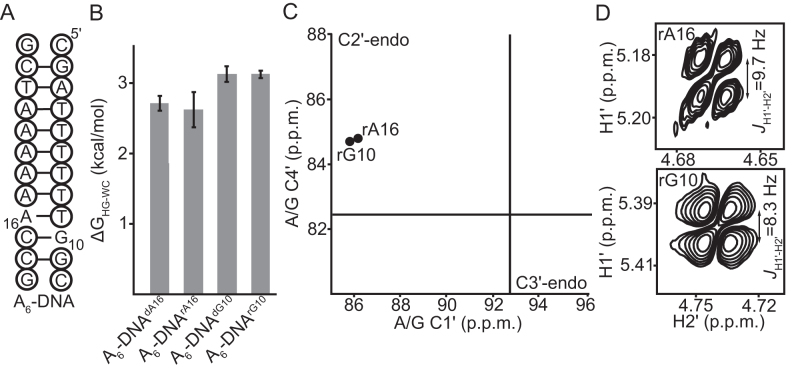
Figure 3.
Addition of a 2′-hydroxyl to purine nucleotides in A6-DNA does not affect sugar geometry and minimally impacts HG bp formation. (A) Secondary structure of A6-DNA with the dNMP residues indicated using black circles. The sites of rNMP incorporation are G10 and A16. (B) The energetic cost of accommodating HG bps relative to WC (ΔGHG-WC) in DNA estimated from melting experiments on A6-DNA constructs with and without N1-methylated purine nucleotides at pH 5.4, 25 mM NaCl and 25°C. Errors in ΔGHG-WC were obtained by propagating the errors from triplicate measurements (see ‘Materials and Methods’). (C) C1′ and C4′ chemical shifts of the rNMPs in A6-DNA fall in the C2′-endo quadrant of a C1′/C4′ correlation plot (77). Black lines denote the average chemical shits of helical A/G residues in RNA as determined from a survey of the BMRB (77). (D) DQF-COSY spectra showing the H1′-H2′ coupling constants of the rNMP sugars in A6-DNA (pH 5.4, 25 mM NaCl and 25°C). The JH1′-H2′ coupling is 8–10 Hz and 0–2 Hz for a C2′-endo and C3′-endo ribose sugar respectively.