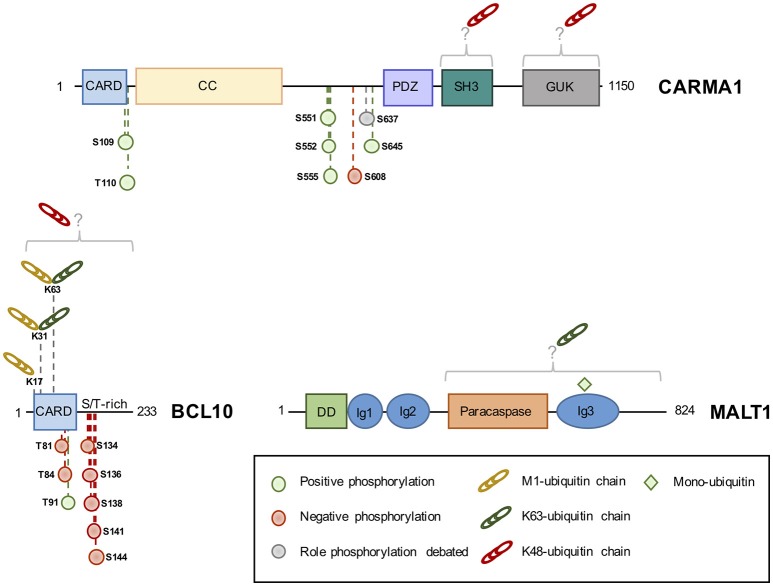
Figure 2.
The domain structure of CBM components and their identified phosphorylation and ubiquitination sites. Phosphorylation of CBM components can positively (green circle) or negatively (red circle) influence NF-κB, although the role of some sites is debated (gray circle). K48-linked ubiquitin linked (red chains) chains lead to protein degradation, while K63 or M1-linked chains (green and yellow chains, respectively) modulate activity. Monoubiquitination (green square) activates MALT1 protease activity. CARD, Caspase recruitment domain; CC, Coiled-Coil; PDZ, PSD95-DLG-ZO1 homology domain; SH3, Src homology 3; GUK, guanylate kinase domain; S/T-rich, serine/threonine-rich domain; DD, death domain; Ig, immunoglobulin-like domain; K, lysine; M, methionine.