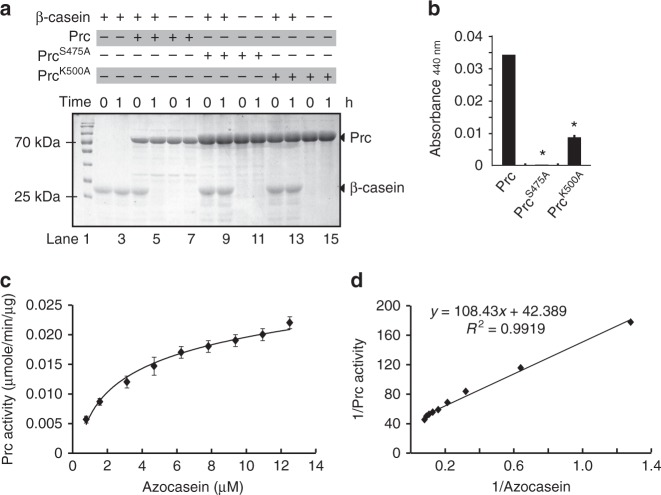
Fig. 2.
Prc is a serine endopeptidase. a Prc degrades β-casein, and its endopeptidase activity is dependent on the Ser475 and Lys500 sites. β-Casein (41.5 μM) was co-incubated with Prc (5 μM) or its recombinant forms (PrcS475A and PrcK500A, 10 μM) at 28 °C for the indicated time. Reactions were stopped and analysed by SDS-PAGE together with Coomassie brilliant blue staining. b Quantification of Prc endopeptidase activity via degradation of the substrate azocasein. Azocasein (424 μM) was mixed with Prc (25 μM), and the reaction was carried out at 28 °C for 30 min. The optical absorbance was measured. Error bars represent standard deviations (n = 3). Asterisks indicate significant differences (Student's t-test, P < 0.05). c and d The Michaelis–Menten kinetics of Prc activity for azocasein hydrolysis. The Michaelis–Menten curve c and Lineweaver–Burk plot d were obtained from the specific reaction velocity of the hydrolysation of azocasein by Prc. The maximum specific Vmax and Km values of the Prc activity were determined from the graphic representations. The data were derived from three independent experiments, and the goodness of fit values (R2) is indicated. a–d, the experiment was repeated three times