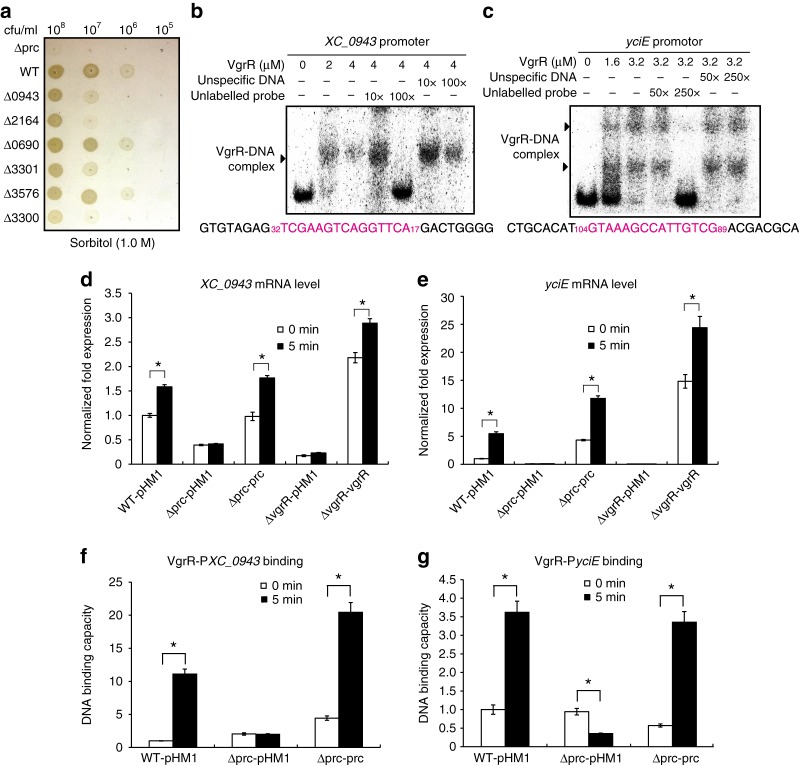
Fig. 8.
Prc regulates the VgrR–promoter-binding interactions in bacterial cells. a Bacterial growth on NYG–sorbitol plate. Bacterial cultures were serially diluted and inoculated onto NYG plus 1.0 M sorbitol plate and grown for 72 h at 28 °C. The experiment was repeated three times. b and c Electrophoretic mobility shift assays revealed that VgrR directly bound the promoter regions of XC_0943 and yciE. PCR products of the promoter regions were labelled with [γ-32P]ATP and used as DNA probes. Unlabelled DNA and non-specific DNA were used as competitors. The sequence of the DNA probe is shown below with the VgrR-binding motif in magenta. Numbers indicate the location relative to the translation initiation site. Each experiment was repeated two times. Triangles indicate VgrR–DNA complexes. d and e The prc mutation caused decreases in the transcription levels of XC_0943 and yciE when bacterial strains were grown under osmostress. qRT-PCR was used to quantify the mRNA levels of these genes in different bacterial strains before and after osmostress stimulation (1.0 M sorbitol, 5 min). Amplification of the cDNA of tmRNA was used as an internal control. A representative of three independent experiments is shown. f and g The prc mutation caused decreases in VgrR–DNA binding in bacterial cells. ChIP-qPCR was conducted to quantify the enrichment of VgrR at the promoter regions of XC_0943 and yciE in vivo when bacterial strains were grown under osmostress conditions (NYG medium plus 1.0 M sorbitol for 5 min). The experiment was repeated three times. In d–g Error bars indicate the standard deviations. Asterisks indicate significant differences of strains before and after osmostress (Student’s t-test, P < 0.05)