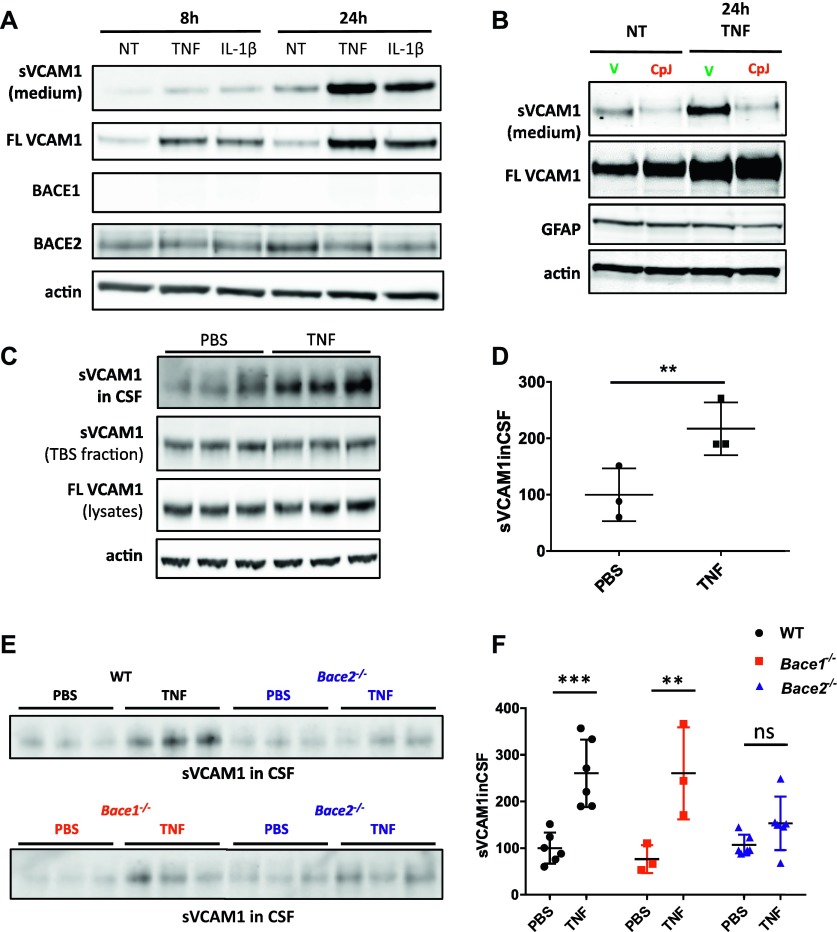
Figure 6. Validation of substrate under pro-inflammatory challenge in vitro and in vivo.
(A) VCAM1 in medium and lysates of primary mixed glia culture treated with murine recombinant TNF (10 ng/ml) or IL-1β (10 ng/ml) for 8 h and 24 h (n = 3). Control blots for BACE1, BACE2, and actin are shown. (B) VCAM1 in medium and lysates of primary mixed glia cells treated with murine recombinant TNF (10 ng/ml) for 24 h, or treated with vehicle or CpJ inhibitor (10 μM) (n = 3). Control blots for GFAP and actin are shown. (C) VCAM1 in triplicates of CSF, TBS fraction, and total cell lysates of cortices from 11-mo-old WT male controls injected with PBS or treated with 250 μg/kg TNF. Actin is used as a loading control. (D) VCAM1 shedding into CSF is up-regulated upon TNF treatment (unpaired t test, P = 0.04; n = 3), whereas no changes are observed in full-length VCAM1 or soluble VCAM1 in TBS fraction shown in (C). (E) VCAM1 in triplicates of CSF of 11-mo-old WT or Bace2−/− mice treated with saline or 250 μg/kg TNF (top panel) and VCAM1 in triplicates of CSF of 11-mo-old Bace1−/− or Bace2−/− mice treated with saline or 250 μg/kg TNF (bottom panel). (F) Two-way ANOVA reveal significant differences in shed VCAM1 between treated and untreated WT and Bace1−/− mice (P = 0.0004 and P = 0.005, respectively; n = 3), but no significant differences in shed VCAM1 between treated and untreated Bace−/− mice (P = 0.69).