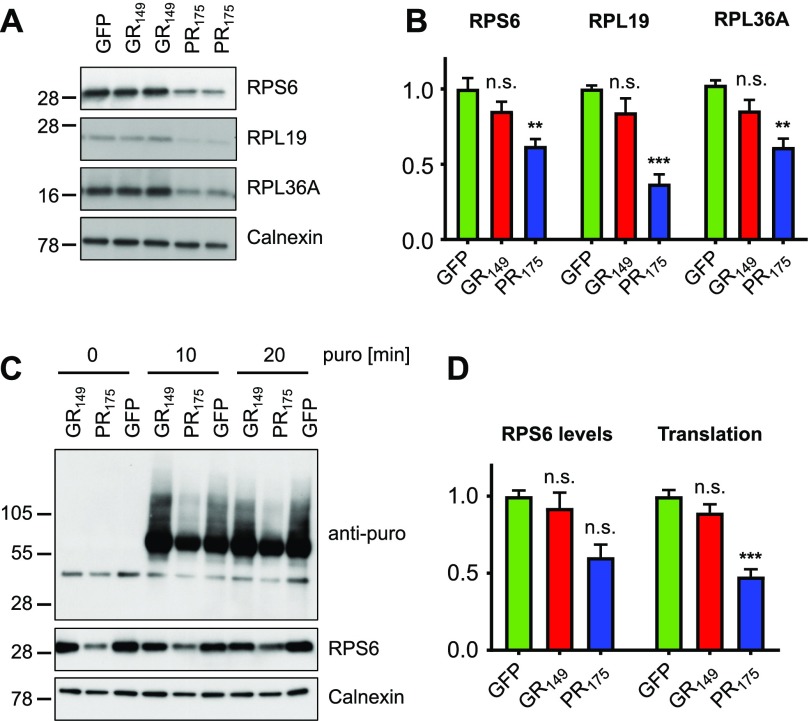
Figure 6. Poly-PR inhibits translation in neurons.
Primary rat cortical neurons (DIV6 + 7) were transduced with GFP, GFP-(GR)149, or (PR)175-GFP lentivirus. (A) Immunoblots show reduced expression of several ribosomal proteins in (PR)175-GFP–expressing neurons. Calnexin was used as loading control. (B) Quantification of RPS6 signal normalized to calnexin (n = 6 from three independent experiments, mean ± SEM, exact P-values: GFP versus GFP-GR149, P = 0.1101 and GFP versus PR175-GFP, P = 0.0010 in one-way ANOVA with Dunnett’s posttest), RPL19 signal normalized to calnexin (n = 6 from three independent experiments, mean ± SEM, exact P-values: GFP versus GFP-GR149, P = 0.1863 and GFP versus PR175-GFP, P = 0.0001 in the Kruskal–Wallis test with Dunn’s posttest), and RPL36A signal normalized to calnexin (n = 6 from three independent experiments, mean ± SEM, exact P-values: GFP versus GFP-GR149, P = 0.1487 and GFP versus PR175-GFP, P = 0.0013 in the Kruskal–Wallis test with Dunn’s posttest). (C) To quantify global translation, primary neurons were incubated with 1 μM puromycin (puro) for 0, 10, and 20 min before sample preparation, which is incorporated into truncated proteins (SUnSET system). A puromycin-specific antibody shows reduced levels of newly synthesized proteins in poly-PR–expressing neurons. Immunoblot for RPS6 and calnexin used as loading control. (D) Quantification of RPS6 signal normalized to calnexin (n = 3, mean ± SEM, Kruskal–Wallis test with Dunn’s posttest, exact P-values: GFP versus GFP-GR149, P = 0.9999 and GFP versus PR175-GFP, P = 0.0507) and puromycin signal normalized to calnexin (n = 6, mean ± SEM, one-way ANOVA with Dunnett’s posttest, exact P-values: GFP versus GFP-GR149, P = 0.2265 and GFP versus PR175-GFP, P = 0.0001).