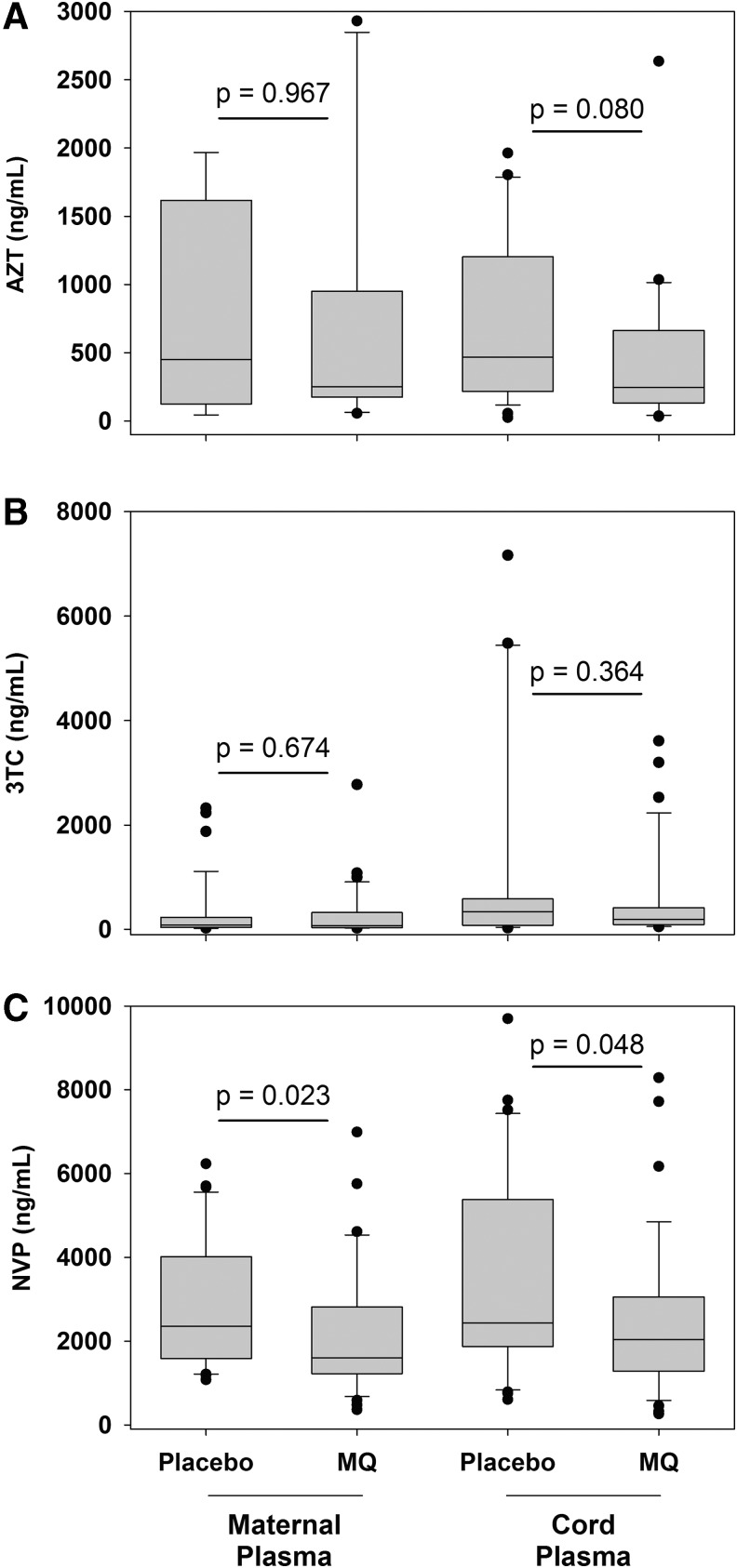
FIG. 1.
Maternal and cord plasma concentrations of zidovudine (AZT), lamivudine (3TC), and nevirapine (NVP) following intermittent preventive treatment with MQ or placebo for malaria control. Zidovudine (AZT) (A), lamivudine (3TC), (B) and nevirapine (NVP) (C) concentrations are presented from maternal plasma (76 placebo study arm, 94 MQ study arm) and cord plasma (74 placebo study arm, 88 MQ study arm) specimens with detectable antiretroviral drug concentrations collected at delivery from HIV-infected pregnant women and their infants. AZT maternal plasma n = 9 (Control), 10 (MQ); cord plasma n = 26 (Control), 28 (MQ); 3TC maternal plasma n = 37 (Control), 39 (MQ); cord plasma n = 23 (Control), 31 (MQ); NVP maternal plasma n = 31 (Control), 34 (MQ); cord plasma n = 30 (Control), and 35 (MQ). MQ, mefloquine.