Abstract
A total of 118 sera were collected during 2016 from two groups of dromedaries from Kebili and Medenine governorates in the south of Tunisia. The aim of this study was to provide the first serological investigation of four emerging vector-borne diseases in two groups of dromedaries in Tunisia. Sera were tested by ELISA and serum neutralisation test to identify West Nile virus (WNV), bluetongue virus (BTV), epizootic haemorrhagic disease virus (EHDV) and Rift Valley fever virus (RVFV). In the first group, the seroprevalence for BTV was 4.6%, while in the second group, it was 25.8% for WNV and 9.7% for BTV. Only serotype 1 was detected for BTV in the two groups. No evidence for circulation of RVF and EHD viruses was revealed. Results indicated that dromedaries can be infected with BTV and WNV, suggesting that this species might play a significant role in the epizootiology of these viral diseases in Tunisia and neighbouring countries.
Introduction
The economic value of the dromedary as a source of meat and transportation with the renewed interest around the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in the Arabian Peninsula raises the question of the impact of the dromedary on new and emergent diseases in North Africa.
The effective control of West Nile (WN), bluetongue (BT), epizootic haemorrhagic disease (EHD) and Rift Valley fever (RVF) depends on a thorough understanding of the possible implications of dromedaries in the replication or the dissemination of the West Nile virus (WNV), bluetongue virus (BTV), epizootic haemorrhagic disease virus (EHDV) and Rift Valley fever virus (RVFV).
Tunisia is located at the interface between the sub-Saharan region and Europe. It has encountered several episodes of emerging vector-borne diseases such as WN, BT and EHD. Tunisia has experienced three major WN epidemics that have particularly affected humans in 1997, 2003 and 2012 (Ben Hassine et al. 2015). In 2015, an equine with clinical signs was reported for the first time in the south of Tunisia in the oases of Tozeur (OIE 2015). Since the first occurrence of BT in 1999, outbreaks have been reported in Tunisia and three serotypes, namely BTV2, BTV1 and BTV4 were identified in 2000, 2006 and 2009, respectively (Hammami 2004; Sghaier et al. 2014). More recently, other BT outbreaks because of serotype 1 in 2011 and serotype 4 in 2013 have been reported (Lorusso et al. 2013; Sghaier et al. 2014). In 2015, a study suggested an active circulation of RVFV and evidence of human exposure in the population of Tunisia was reported (Bosworth et al. 2016). EHDV was detected for the first time in Tunisia in 2006. Recently, clinical cases in cattle were reported in countries surrounding the Mediterranean Basin, including Morocco, Algeria and Tunisia, with the emergence of EHDV serotype 6 (Ben Dhaou et al. 2016). The aim of the present study was to provide the first serological investigation of four emerging vector-borne diseases in two groups of dromedaries in Tunisia with a focus on WNV, BTV, EHDV and RVFV.
Materials and methods
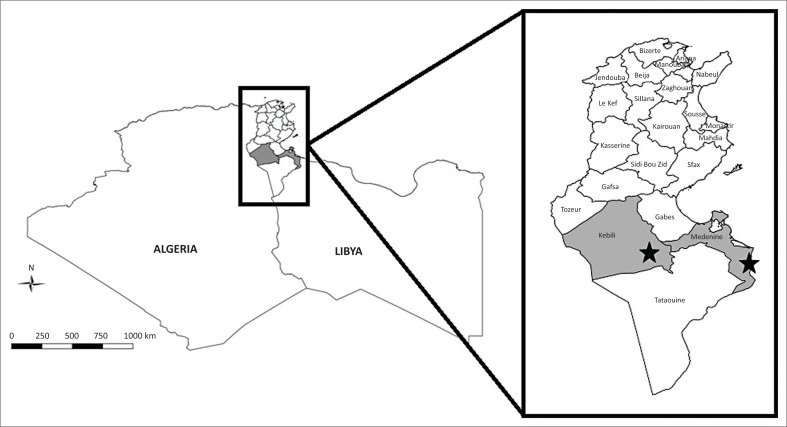
This study was carried out in 2016 in the governorates of Kebili and Medenine (Figure 1). These two governorates were selected because of their relatively high dromedary density and cross-border animal movement within the region (Ayari-Fakhfakh et al. 2011). These factors represent high-risk zones for disease transmission in Tunisia.
FIGURE 1.
Geographical location of sampled dromedaries in the south of Tunisia (Kebili and Medenine governorates).
The first lot of 87 sera was collected from dromedaries intercepted at the country border posts by the Tunisian veterinary services in the region of Ben Guerden (governorate of Medenine) where the dromedaries usually circulate in the arid desert. The second lot of 31 sera was collected from individual dromedaries in contact with sheep herds in the oases of Kebili, which are characterised by traditional irrigation where the main practice is flooding (Mekki et al. 2013). Sera were tested by enzyme-linked immunosorbent assay (ELISA) for WNV, BTV, RVFV and EHDV. IgG antibodies to RVFV were detected using ID Screen® RVF competition multi-species ELISA kits (ID Vet; Innovative Diagnostics, Montpellier, France). For EHD, all the sera were tested by a blocking competitive ELISA (LSI VET EHDV Blocking, LSI, France) to detect the specific anti-EHDV VP7 antibodies. Sera were screened for the presence of group-specific BTV antibodies by using the c-ELISA IDEXX Bluetongue Competition® assay (IDEXX BT, Hoofddorp, the Netherlands). The Usutu virus (USUV)- and WNV-specific antibodies were detected using a commercially available competition ELISA, (ID Screen® West Nile Competition, ID Vet, Montpellier, France). Serum neutralisation (SN) test for 26 BTV serotypes and for WNV–USUV was used on positive samples (Di Gennaro et al. 2014). The test was conducted at the Istituto Zooprofalitico Sperimentale dell’Abruzzo e del Molise ‘G. Caporale’ (IZSAM) and OIE reference laboratory for BTV and WND, under biosafety level 3 conditions as previously described (OIE 2016).
Results and discussion
Seropositivity of BTV was detected in the two groups (4.6% in the group of Medenine vs. 9.7% in the group of Kebili). This study confirms the circulation of serotype 1 in Tunisia that was detected for the last time in 2011 (Sghaier et al. 2014). For BTV, Tunisia has adopted a vaccination strategy in sheep using a bivalent vaccine (BTV1 and BTV4), but dromedaries are not vaccinated. Three species of Culicoïdes were predominantly detected in the south of Tunisia (Gabes governorate): Culicoïdes jumineri, Culicoïdes sahariensis and Culicoïdes submarimitimus although Culicoïdes imicola is considered the main vector of BTV (Mellor 1996; Sghaier et al. 2009). According to Batten et al. 2011, dromedaries seem to act as reservoirs, possibly playing a role in the spread of the disease by helping the virus to get through the geographic barrier which is represented by the Sahara desert. This desert stands between the tropics and subtropics, where BTV is endemic, and North Africa, where it periodically causes epizootics. However, there is scarce information about the clinical manifestations of BT in dromedaries. South American camels are susceptible to BTV infection, but they develop only a mild form of the disease (Schulz et al. 2012). For WNV, this study revealed that 25.8% of the dromedaries, which live in oases, were seropositive. This result is consistent with the findings reported by Ben Hassine et al. (2015), where a high seropositivity in horses was found in the oases of Kebili that were identified as high-risk areas for WNV circulation in Tunisia. Moreover, the presence of Culex pipiens in the south of Tunisia and isolation of WN virus from C. pipiens in central Tunisia (lineage 1) confirms this finding (Wasfi et al. 2016). Recent studies in North Africa reported WNV seropositivity rates in dromedaries ranging from 13% (Touil et al. 2012) to 29% (El-Harrak et al. 2011). Similar to human and horse populations, in which less than 1% of WNV-infected individuals become severely ill, it appears that the majority of camels infected with WNV are asymptomatic and recover uneventfully. No evidence for the circulation of RVF and EHD viruses are revealed in this study. Serologic evidence of RVF in dromedaries is frequently reported (Swai & Sindato 2015), yet the description of clinical signs is rare. Subclinical, mild forms and healthy carriers of the virus (Paweska 2015; Swanepoel & Coetzer 2004) have been reported. The presence of the RVF competent vector C. pipiens and Aedes caspius in oases make this ecosystem favourable for RVF transmission in Tunisia where Aedes could be responsible for the initiation of an outbreak and Culex maintains the virus activity (Soti et al. 2012). For EHDV, dromedaries seem not to be involved in the disease transmission. The study conducted by Wernery et al. (2013) using ELISA test showed that 29% of dromedaries from the United Arab Emirates have antibodies to EHDV.
Conclusion
This study, for the first time, reports infection of dromedaries with WNV and BTV in Tunisia, confirming the reported infections of dromedaries with BTV and WNV in North Africa and the Middle East. A larger serosurvey with an entomological monitoring and a syndromic surveillance in a One Health concept is needed to understand the implications of dromedaries in the transmission of emerging arthropod-borne viruses which can be very useful for the purposes of control and planning surveillance activities not only in Tunisia but also for all the regions of North Africa.
Acknowledgements
This study was partially funded by IAEA (RAF50/68 regional project), Italian Ministry of Health IZS AM 01/10 RC and EU grant FP74613996 Vmerge, and is catalogued by the Vmerge Steering Committee as VMerge0011. The authors would like to thank SPANA-Tunisia for logistical support.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
S.H., T.B.H. and J.A. conceived and designed the experiments. T.B.H., J.A., S.H. and S.S. wrote the article. I.B.S., W.C., F.M., G.S., S.S. and K.B.H. prepared the samples and carried out laboratory analysis. S.H., T.B.H., J.A., S.S., G.S. and F.M. reviewed the article.
Footnotes
How to cite this article: Hassine, T.B., Amdouni, J., Monaco, F., Savini, G., Sghaier, S., Selimen, I.B. et al., 2017, ‘Emerging vector-borne diseases in dromedaries in Tunisia: West Nile, bluetongue, epizootic haemorrhagic disease and Rift Valley fever’, Onderstepoort Journal of Veterinary Research 84(1), a1316. https://doi.org/10.4102/ojvr.v84i1.1316
Note: T.B.H. and J.A. both contributed equally to this manuscript.
References
- Ayari-Fakhfakh E., Ghram A., Bouattour A., Larbi I., Gribâa-Dridi L., Kwiatek O. et al. , 2011, ‘First serological investigation of peste-des-petits-ruminants and Rift Valley fever in Tunisia’, Veterinary Journal 187(3), 402–404. https://doi.org/10.1016/j.tvjl.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Batten C.A., Harif B., Henstock M.R., Ghizlane S., Edwards L., Loutfi C. et al. , 2011, ‘Experimental infection of camels with bluetongue virus’, Research in Veterinary Science 90(3), 533–535. https://doi.org/10.1016/j.rvsc.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Ben Dhaou S., Sailleau C., Babay B., Viarouge C., Sghaier S., Zientara S. et al. , 2016, ‘Molecular characterisation of epizootic haemorrhagic disease virus associated with a Tunisian outbreak among cattle in 2006’, Acta Veterinaria Hungarica 64(2), 250–262. https://doi.org/10.1556/004.2016.025 [DOI] [PubMed] [Google Scholar]
- Ben Hassine T., Conte A., Calistri P., Candeloro L., Ippoliti C., De Massis F. et al. , 2015, ‘Identification of suitable areas for West Nile virus circulation in Tunisia’, Transboundary and Emerging Diseases. https://doi.org/10.1111/tbed.12384 [DOI] [PubMed] [Google Scholar]
- Bosworth A., Ghabbari T., Dowall S., Varghese A., Fares W., Hewson R. et al. , 2015, ‘Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia’, New Microbes and New Infections 9, 1–7. https://doi.org/10.1016/j.nmni.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro A., Lorusso A., Casaccia C., Conte A., Monaco F. & Savini G, 2014, ‘Serum-neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile Virus antibodies in human and animal serum samples’, Clinical and Vaccine Immunology 21(10), 1460–1462. https://doi.org/10.1128/CVI.00426-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Harrak M., Martín-Folgar R., Llorente F., Fernández-Pacheco P., Brun A., Figuerola J. et al. , 2011, ‘Rift Valley and West Nile Virus antibodies in camels, North Africa’, Emerging Infectious Diseases 17(12), 2372–2374. https://doi.org/10.3201/eid1712.110587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami S, 2004, ‘North Africa: A regional overview of bluetongue virus, vectors, surveillance and unique features’, Veterinaria Italiana 40(3), 43–46. [PubMed] [Google Scholar]
- Lorusso A., Sghaier S., Carvelli A., Di Gennaro A., Leone A., Marini V. et al. , 2013, ‘Bluetongue virus serotypes 1 and 4 in Sardinia during autumn 2012: New incursions or re-infection with old strains?’, Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases 19, 81–87. [DOI] [PubMed] [Google Scholar]
- Mekki I., Jacob F., Marlet S. & Ghazouani W, 2013, ‘Management of groundwater resources in relation to oasis sustainability: The case of the Nefzawa region in Tunisia’, Journal of Environmental Management 121, 142–151. https://doi.org/10.1016/j.jenvman.2013.02.041 [DOI] [PubMed] [Google Scholar]
- Mellor P.S, 1996, ‘Culicoides: Vectors, climate change and disease risk’, Veterinary Bulletin 66, 1–30. [Google Scholar]
- Office International des épizooties , 2015, Animal health information, viewed 04 March 2016, from http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary
- Office International des épizooties , 2016, Manuel des tests de diagnostic et des vaccins pour les animaux terrestres 2016, viewed 03 March 2016, from http://www.oie.int/fr/normes-internationales/manuel-terrestre/acces-en-ligne/2016
- Paweska J.T, 2015, ‘Rift Valley fever’, Revue Scientifique Et Technique (International Office of Epizootics) 34(2), 375–389. https://doi.org/10.20506/rst.34.2.2364 [DOI] [PubMed] [Google Scholar]
- Schulz C., Eschbaumer M., Rudolf M., König P., Keller M., Bauer C. et al. , 2012, ‘Experimental infection of South American camelids with bluetongue virus serotype 8’, Veterinary Microbiology 154(3–4), 257–265. https://doi.org/10.1016/j.vetmic.2011.07.025 [DOI] [PubMed] [Google Scholar]
- Sghaier S., Hammami S., Dkhil A. & Delécolle J.C, 2009, ‘Entomological surveillance of Culicoides (diptera: Ceratopogonidae), vector of Bluetongue in Tunisia’, Revue d’élevage et de médecine vétérinaire des pays tropicaux 62, 2–4. [Google Scholar]
- Sghaier S., Leone A., Orsini M., Marcacci M., Teodori L., Scipioni G. et al. , 2014, ‘Whole genome analysis of a BTV-4 strain isolated in Tunisia in 2013’, Bluetongue and Related Orbiviruses IV International Conference, Rome, Italy, November 05–07, 2014. [Google Scholar]
- Soti V., Tran A., Degenne P., Chevalier V., Lo Seen D., Thiongane Y. et al. , 2012, ‘Combining hydrology and mosquito population models to identify the drivers of Rift Valley fever emergence in semi-arid regions of West Africa’, PLoS Neglected Tropical Diseases 6(8). https://doi.org/10.1371/journal.pntd.0001795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai E.S. & Sindato C, 2015, ‘Seroprevalence of Rift Valley fever virus infection in camels (dromedaries) in northern Tanzania’, Tropical Animal Health and Production 47(2), 347–352. https://doi.org/10.1007/s11250-014-0726-y [DOI] [PubMed] [Google Scholar]
- Swanepoel R. & Coetzer J.A.W, 2004, ‘Rift Valley fever’, in Coetzer J. & Tustin R. (eds.), Infectious diseases of livestock, pp. 1037–1070, 2nd ed., Oxford University Press, Oxford. [Google Scholar]
- Touil N., Cherkaoui Z., Lmrabih Z., Loutfi C., Harif B. & El Harrak M, 2012, ‘Emerging viral diseases in dromedary camels in the Southern Morocco’, Transboundary and Emerging Diseases 59(2), 177–182. https://doi.org/10.1111/j.1865-1682.2011.01282.x [DOI] [PubMed] [Google Scholar]
- Wasfi F., Dachraoui K., Cherni S., Bosworth A., Barhoumi W., Dowall S. et al. , 2016, ‘West Nile virus in Tunisia, 2014: First isolation from mosquitoes’, Acta Tropica 159, 106–110. https://doi.org/10.1016/j.actatropica.2016.03.037 [DOI] [PubMed] [Google Scholar]
- Wernery U., Thomas R., Raghavan R. & Syriac G, 2013, ‘Serological evidence of epizootic haemorrhagic disease and Schmallenberg virus in dromedaries’, Journal of Camel Practice and Research 20(2), 135–137. [Google Scholar]