Table 1. Optimization of the reaction conditions a .
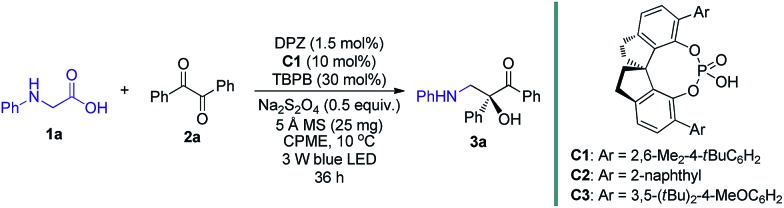
| |||
| Entry | Variation from standard conditions | Yield b (%) | ee c (%) |
| 1 | 0.5 mol% DPZ in CH2Cl2 at 25 °C and without C1 and any additives, 12 h | 62 | N.A. |
| 2 | None | 78 | 93 |
| 3 | C2 instead of C1 | 74 | 76 |
| 4 | C3 instead of C1 | 73 | 67 |
| 5 | Ru(bpy)3Cl2·6H2O instead of DPZ | 58 | 89 |
| 6 | Rose Bengal instead of DPZ | 69 | 91 |
| 7 | No TBPB | 76 | 89 |
| 8 | No Na2S2O4 | 75 | 90 |
| 9 | No 5 Å MS | 76 | 90 |
| 10 | No DPZ | 35 | 88 |
| 11 | No light | 0 d | N.A. |
| 12 | Under air | 0 e | N.A. |
| 13 | No C1 | 53 | N.A. |
aReaction conditions: 1a (0.075 mmol), 2a (0.05 mmol), degassed CPME (1.0 mL), 10 °C, irradiation with blue LED (3 W, 450 nm), 36 h.
bYield of the isolated product.
cDetermined by HPLC analysis on a chiral stationary phase.
dNo reaction was detected.
e 1a was consumed, but no 3a was obtained. TBPB = tetra-n-butylphosphonium bromide. CPME = cyclopentyl methyl ether. N.A. = not available.
